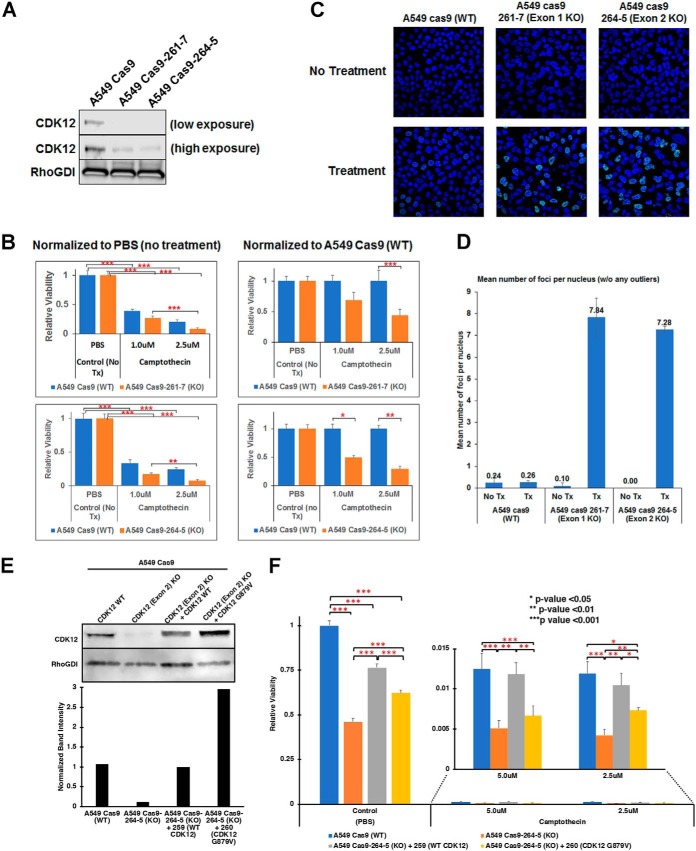
Fig. 7.
CRISPR-Cas9-mediated knockdown of CDK12 increases chemotherapy sensitivity in A549 human lung adenocarcinoma cells. A, Western blotting of CDK12 in parental A549-Cas9 control cells and stable clones expressing CRISPR sgRNA targeting exon 1 (261-7) and exon 2 (264–5). CDK12 expression is ablated in both clones. B, Camptothecin sensitivity of the parental and CDK12 knockdown cell lines. The data is normalized to mock (PBS) treatment (left) or the with parental control cells (right) (*** p < 0.001; ** p < 0.01; * p < 0.05). C–D, γH2AX foci formation on treatment with camptothecin (C) and their quantitation (D) in parental and CDK12 exon 1 and exon 2 knockout cells. E, Wild type CDK12 and CDK12-G879V mutant were overexpressed in CDK12 exon 2 knockout cells for rescue experiments. Western blotting for CDK12 expression (upper panel) shows overexpression of corresponding CDK12 protein in the CDK12 exon 2 knockout cells. Rho-GDI was used as a loading control and relative quantitation of CDK12 expression is shown in lower panel. F, Camptothecin sensitivity of CDK12 exon 2 knockout cells compared with parental cells and its rescue by wild type CDK12, but not CDK12-G879V mutant (*** p < 0.001; ** p < 0.01; * p < 0.05).