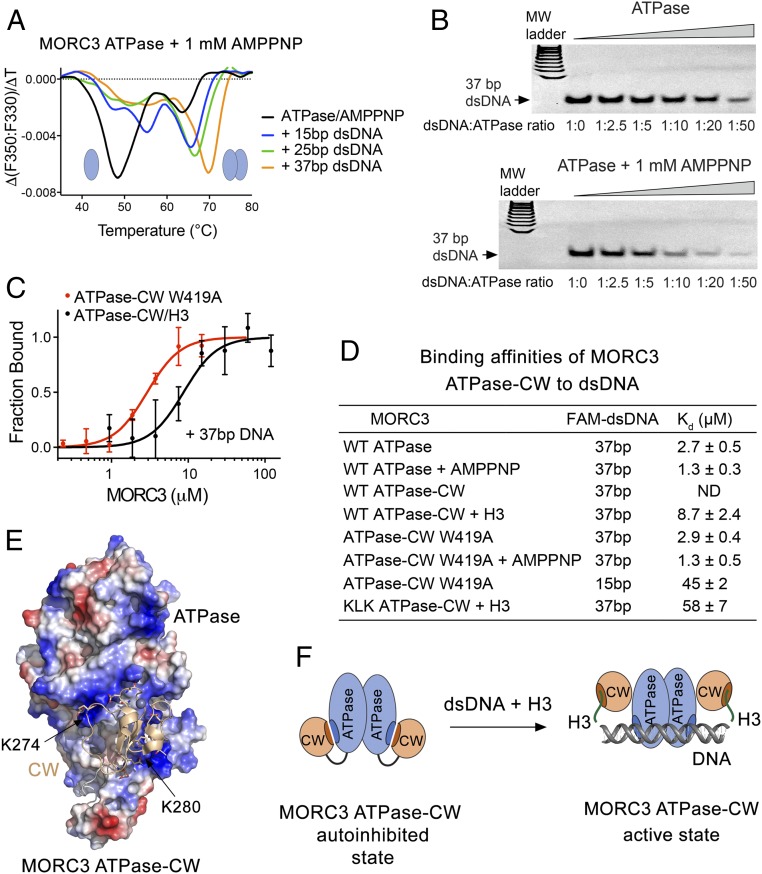
Fig. 4.
DNA-binding property of MORC3 ATPase–CW. (A) MORC3 ATPase binding to the indicated double-stranded DNA fragments in the presence of AMPPNP monitored by DSF. (B) EMSA with 37-bp dsDNA in the presence of increasing amounts of ATPase with or without AMPPNP. (C) Binding curves used to determine binding affinities of the MORC3 ATPase–CW W419A mutant for DNA by MST. Error represents SD between three separate experiments. (D) Binding affinities of the MORC3 ATPase or ATPase–CW cassette, WT and mutants, to the indicated dsDNAs; Kd values ± SD are shown. ND, not detected. (E) Electrostatic surface potential of the ATPase domain (in the ATPase–CW cassette) is colored blue and red for positive and negative charges, respectively. The CW domain is shown as a ribbon with selected negatively charged D and E residues depicted in stick form. (F) A model of MORC3 activation through the bivalent synergistic interactions with H3 and DNA.