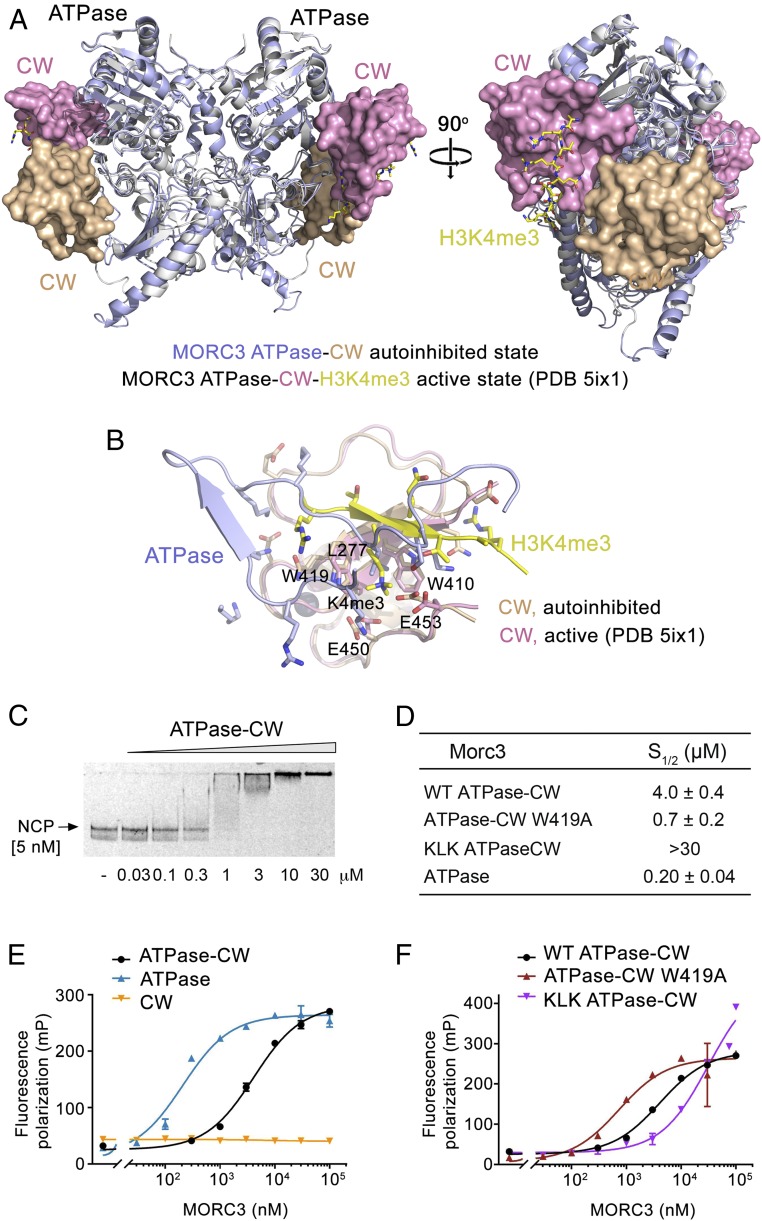
Fig. 5.
MORC3 ATPase–CW binds to the nucleosome. (A) Structural overlay of the autoinhibited (this study; light blue and wheat) and active (PDB ID code 5ix1; white and magenta) (11) states of the MORC3 ATPase–CW cassette. The ATPase and CW domains are shown in ribbon and surface representations, respectively. H3K4me3 peptide in the active state of MORC3 is shown in stick form (yellow). (B) Structural overlay of the CW domain in the autoinhibited state (wheat) bound to the α8–β8 and β8–β9 loops of the ATPase domain (light blue) with the CW domain in the active state (PDB ID code 5ix1; magenta) bound to the H3K4me3 peptide (yellow). (C) EMSA with NCPs in the presence of increasing amounts of ATPase–CW. (D–F) Binding affinities (D) and binding curves (E and F) for the interactions of the indicated MORC3 constructs with NCP as measured by fluorescence polarization. Error bars represent an SD based on three separate experiments.