Significance
A replicated management experiment was conducted across >90,000 km2 to test recovery options for woodland caribou, a species that was functionally extirpated from the contiguous United States in March 2018. Recovery options were reductions of predators, reductions of overabundant prey, translocations, and creating fenced refuges from predators. Population growth was strongest where multiple recovery options were applied simultaneously. This adaptive management study was one of the largest predator-prey manipulations ever conducted and provided positive results for this endangered North American ungulate.
Keywords: adaptive management, conservation, predator-prey dynamics, apparent competition, ecosystem experiment
Abstract
Adaptive management is a powerful means of learning about complex ecosystems, but is rarely used for recovering endangered species. Here, we demonstrate how it can benefit woodland caribou, which became the first large mammal extirpated from the contiguous United States in recent history. The continental scale of forest alteration and extended time needed for forest recovery means that relying only on habitat protection and restoration will likely fail. Therefore, population management is also needed as an emergency measure to avoid further extirpation. Reductions of predators and overabundant prey, translocations, and creating safe havens have been applied in a design covering >90,000 km2. Combinations of treatments that increased multiple vital rates produced the highest population growth. Moreover, the degree of ecosystem alteration did not influence this pattern. By coordinating recovery involving scientists, governments, and First Nations, treatments were applied across vast scales to benefit this iconic species.
The late Graeme Caughley emphasized that naturally rare yet broadly distributed species are the most challenging to conserve (1). These organisms will overlap with many other valuable natural resources, creating the potential for substantial socioeconomic conflict. Such large-landscape species also encompass many ecological scales, inherently leading to increased uncertainty (2). Scientists have increasingly called for management experiments to help resolve such uncertainty (3), but the challenge has been to apply treatments at sufficiently broad scales of space and time to include relevant ecosystem processes. This approach is referred to as adaptive management and is predicated on creating lasting partnerships between scientists and resource managers to test alternative hypotheses using contrasting policies (4–6).
Adaptive management was initially intended to guide the sustainable consumption of natural resources, such as fisheries or wood fiber (4). But can this method be successfully applied to recovering endangered species? Many have argued that it can, but examples are rare (7, 8). We highlight this approach using perhaps the greatest terrestrial conservation challenge in North America: recovering woodland caribou (Rangifer tarandus caribou). These animals live across 3 million km2 from Alaska to Newfoundland, and their critical habitat overlaps petroleum deposits and forest stands worth billions of dollars (9). Caribou are also a key umbrella species for boreal biodiversity, and their range covers one of the largest carbon stores on the planet—the boreal forest (10). Most populations are in decline and extirpation is ongoing (11, 12), setting the stage for an unparalleled conflict between conservation and natural-resource economies (9). With three barren females remaining in the only population south of the 49th parallel, caribou are the first large-mammal extirpation in recent history from the contiguous United States (13).
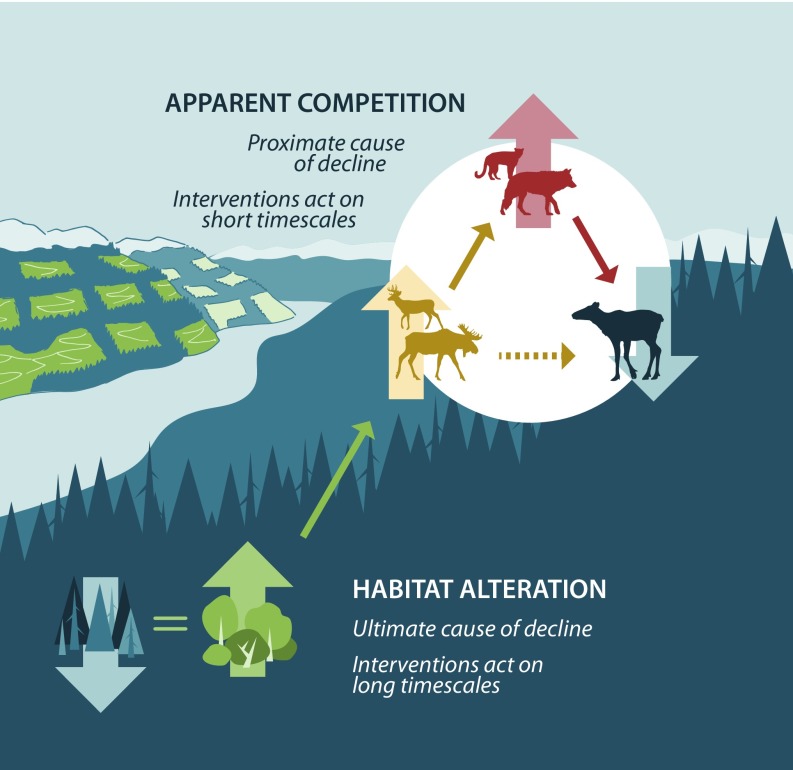
The complexity of this problem is the result of broad alterations to ecosystem dynamics across three trophic levels: vegetation, herbivores, and carnivores (14, 15) (Fig. 1). Even under pristine conditions, caribou are less fecund than deer (Odocoileus virginianus) or moose (Alces alces) (16) and can be more vulnerable once encountered by predators (17). Yet, in human-altered systems, the creation of productive, early seral forests buoy primary prey numbers such as moose and deer (18, 19). Thence, predator numbers are maintained by the more numerous moose and deer (20, 21), creating a decoupling between predator numbers and caribou. Consequently, caribou can decline to extinction while predators are maintained by generalist herbivores (14, 22). This process is referred to as apparent competition (23) and affects many threatened taxa (24), especially as climate and land-use change facilitate the spread of generalist prey. In the well-known case of California’s Channel Island fox (Urocyon littoralis), invasive feral pigs (Sus scrofa) subsidized predatory golden eagles (Aquila chrysaetos), causing declines in this endangered fox (25, 26). Recovery was achieved by the simultaneous reduction of pigs and eagles. In that case, the subsidy of overabundant prey could be reversed relatively quickly. For woodland caribou, however, subsidies of prey will last for decades because of long-term changes to forest age distributions (Fig. 1). Therefore, the classic solution of protecting remaining critical habitat (27) will not save most caribou populations because of the time needed to recover old forests and the continental scale of disturbance (28). In such cases, population management is needed until protection and recovery of habitat overcome the legacy of industrial development. Population-based recovery measures include direct predator reductions (29), prey reductions that lead to fewer predators (30, 31), animal translocations, and the creation of short-term safe havens from predators (predator-proof fences, i.e., maternal pens). Reducing predators can produce immediate benefits (29, 32–34) but can be unpopular because it is a proximate, short-term solution (35). Reducing subsidized prey is one trophic level closer to the ultimate cause, and safe havens are small (<10 ha) fenced areas that exclude predators and protect caribou during the calving season.
Fig. 1.
Process of apparent competition [AC; (23)] spanning three trophic levels: vegetation, herbivores, and carnivores. AC occurs between abundant primary prey (moose and deer) and endangered woodland caribou. In this instance, the early seral forests will last for decades, facilitating the subsidy of primary prey. Therefore, immediate management of large mammals (herbivores and carnivores) is required to recover caribou until the early seral forests transition to closed canopies. Image courtesy of Kate Broadley (Fuse Consulting, Alberta, Canada).
Here we contrast management experiments designed to reduce uncertainty about how to conserve endangered caribou. The primary hypothesis was that population declines could be reversed by removing the proximate limiting factor, excessive predation, because broad-scale ecosystem restoration would take decades to achieve. We included early seral forest (36) as a covariate to test the alternate hypothesis that the degree of ecosystem alteration would influence population response (27, 37). This design essentially contrasts the proximate limiting factor of predation with the ultimate factor of ecosystem alteration. We also qualitatively evaluated how the intensity of treatments and population size affected recovery. The population treatments covered large areas (3,000–8,500 km2) and included predator removal (wolves; n = 6), subsidized-prey reduction (n = 4), predator removal plus safe havens (n = 1), and translocations of caribou (n = 1). These were compared with six untreated, control populations. Our synthesis revealed three conclusions that credibly inform recovery for caribou and other endangered species. First, an adaptive management framework, with control populations, was critical to determining if population growth increased following a specific treatment. Second, a treatment had to be applied intensively to produce a measurable effect. Third, applying two treatments simultaneously produced an additive effect on caribou population growth.
Results
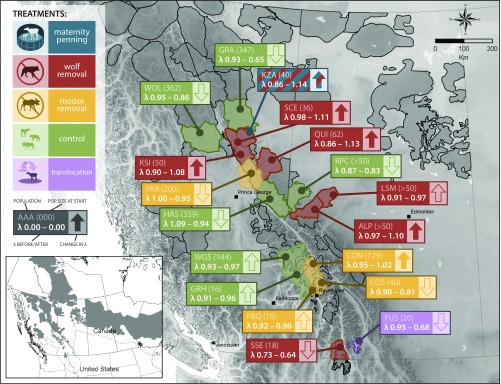
We compared the population growth rate (λ) of 12 caribou populations before and after a treatment as well as 6 adjacent populations used as experimental controls. Before treatments, 16 of 18 populations were in decline (λ < 1; Fig. 2). After treatments began, 8 of 12 treated populations showed λ increases of 0.04–0.28, and 6 of these 8 achieved stable or increasing λ (λ ≥ 1). None of the control populations had positive population growth during treatments. The most pronounced increase occurred within the Klinse-Za (KZA) population (λ = 0.86–1.14), where the combination of wolf removal plus maternal penning resulted in a near-doubling of population size, from 36 to 67 animals between 2013 and 2018 (SI Appendix, Table S1). The adjacent control populations, Graham (GRA) and Wolverine (WOL), continued to decline at λ = 0.65 and 0.86 (Fig. 2).
Fig. 2.
Population growth rates (λ; 1 = stability) before and after treatments were initiated, with controls matched by a similar time period (SI Appendix, Table S1). Solid arrows indicate λ > 1. Population values apply to the beginning of treatment. Black outlines show woodland caribou range boundaries. (Inset) current (gray) and historic (dashed line) distribution in the contiguous United States and Canada. ALP, À la Pêche; CON, Columbia North; COS, Columbia South; FBQ, Frisby Queest; GRA, Graham; GRH, Groundhog; HAS, Hart South; KSI, Kennedy Siding; KZA, Klinse-Za; LSM, Little Smoky; PAR, Parsnip; PUS, Purcells South; QUI, Quintette; RPC, Redrock–Prairie Creek; SCE, Scott East; SSE, South Selkirks; WGS, Wells Gray South; WOL, Wolverine.
An ANCOVA revealed that the effect of treatment (five levels; Table 1) explained 44.2% of the variation in change to λ (Δλ), with positive effects for wolf reduction and wolf reduction + penning. Percentage alteration of forest cover explained only 4.2% of the variation in Δλ (SI Appendix, Fig. S1 and Table S2). The ANCOVA with both treatment and forest alteration was less parsimonious and explained less variation (ΔAICc = 4.68, R2 = 0.42; see SI Appendix, Table S3) than the effect of treatment alone. Six of the treated populations numbered <50 animals at the start of a treatment, and only one of these (KZA) achieved positive population growth (λ = 1.14) when subjected to two treatments simultaneously. Only two of the larger treated populations (>50 animals) did not achieve an increased λ following treatments: Parsnip (PAR) and À la Pêche (ALP). Both had low intensity of management applied (SI Appendix, Table S1). In PAR, moose were reduced by 40% compared with Columbia North (CON), where moose were reduced by >80% and λ increased by 0.064–1.02. In ALP, wolf reduction was applied only to the winter range during the first eight years of treatment and λ did not increase. The treatment was then expanded to the entire range for three years and λ increased from 0.92 to 1.10 (SI Appendix, Table S1). The US/Canada transboundary South Selkirks (SSE) population was small (n = 18) when wolf removal was initiated and expanded only to the Canadian portion of the range (Fig. 2); the population declined from 18 to 3 barren females as of March 2018. In summary, caribou λ did not respond in the three herds with low treatment intensity (SSE, PAR, and ALP), but when ALP transitioned from low to high intensity, λ increased from 0.92 to 1.10. Finally, the translocation of 20 animals to Purcells South (PUS) in 2012 did not improve λ, with only 4 remaining animals in March 2018.
Table 1.
Analysis of covariance explaining change in λ (Δλ) based on treatments for woodland caribou
| Factor | Estimate | SE | t value | P value |
| Intercept | −0.093 | 0.056 | −1.642 | 0.125 |
| Treatment level | ||||
| Moose reduction | 0.079 | 0.089 | 0.891 | 0.389 |
| Wolf reduction | 0.220 | 0.080 | 2.763 | 0.016 |
| Wolf reduction and Penning | 0.372 | 0.149 | 2.496 | 0.027 |
| Translocation | −0.232 | 0.149 | −1.553 | 0.144 |
Intercept represents control populations. Multiple R2 = 0.57; adjusted R2 = 0.44. Analysis was performed on change in r, where r = ln (λ). Less parsimonious models are presented in the SI Appendix, Tables S2 and S3.
Discussion
By focusing on the ultimate recovery metric, caribou population growth, we demonstrated clear benefits of an adaptive management framework applied to endangered species over an enormous landscape. Reducing one limiting factor improved λ, but the greatest increase occurred when two limiting factors were reduced simultaneously. The implementation of wolf reductions followed by penning within KZA illustrates the iterative nature of adaptive management. Given that penning is designed to increase recruitment and wolf reduction increases adult survival, implementing both achieved the highest λ. And critically, pairing populations experiencing treatments with controls that received no similar recovery actions strengthened our inferences.
Intensity of treatment, both numerically and spatially, was a key factor in detecting a population response. In all three instances where treatment intensity was limited, no caribou response was observed. These results follow previous studies suggesting that predation rates should not change linearly with prey density, partially because of density-dependent processes (31, 38, 39). Indeed, caribou in both the PAR moose reduction and the associated Hart South (HAS) control continued to decline, likely because moose were reduced by only 40%. Similarly, when wolves were reduced over just a portion of ALP and SSE, caribou λ did not improve. But when the treatment was adaptively expanded to the entire range of ALP, λ increased substantially. Conclusions from these actions are becoming clear—half measures erode public confidence when the outcome is unlikely to achieve recovery. Resources should be directed strategically and toward recovery treatments of sufficient intensity to achieve results. Finally, as with many translocations (40), moving 20 caribou to PUS was unsuccessful because most of these animals were shortly killed by predators (41), driving home Caughley’s primary message of first removing agents of decline before attempting such actions (1).
The appeal of adaptive management lies with the simple logic of using management actions to test a hypothesis and, if possible, to test alternate hypotheses with contrasting policies (4, 6). These actions should follow detailed modeling of the system to help minimize risks of unintended consequences (3, 31, 42) but also to refute or validate conceptual models of ecosystem dynamics. For example, previous theory suggested caution when removing subsidized prey because of demographic time lags of predators and depensatory predation that can exacerbate declines of rare prey (31, 38). An empirical example occurred within our system when deer populations crashed in 1997 and cougars (Puma concolor) switched to eating caribou (see ref. 31). This information must be adaptively incorporated into recovery plans, but can create imbalances in study designs and implementation. In our case, the lack of replication for some treatments—for example, translocations—may weaken inferences. However, when considered in light of independent studies indicating that animal translocations often fail (40), even with caribou (43), inferences are consistent. Similarly, the combination of treatments (penning and wolf reduction in KZA) can make it challenging to definitively conclude which treatment was strongest. Indeed, balanced and replicated factorial experiments are a laudable goal, but we agree with Krebs’ (44) synthesis of Caughley’s perspective on uncertainty in conservation (1): “Several suspected agents of decline may have to be removed at once… It is better to save the species than to achieve scientific purity.” We hope this approach will encourage others to pursue a priori planned designs or retrospective approaches to adaptive management. Nonetheless, social and logistical barriers to implementation are immense, primarily due to real or perceived impacts on human values (4). Consequently, according to Westgate et al. (7), only 1% of studies that have attempted adaptive management report any response metrics. The plight of woodland caribou has likely reduced these barriers, enabling partnerships across political jurisdictions, among academics, First Nations, managers, industry, and conservationists (45).
The global spread of generalist species through habitat modification and climate change (46) will continue to exacerbate the endangerment and extirpation of species via complex ecological mechanisms such as apparent competition. In many cases, recovery will involve the reduction of expanding prey or abundant native predators. Although six caribou populations grew within highly disturbed landscapes, intensive management was required to achieve this outcome. Support for direct predator reduction is likely to wane (35) unless the ultimate cause of decline, habitat alteration, is addressed. In the case of caribou, like many other endangered species, anthropogenic alterations of forested ecosystems are the ultimate cause of declines. Habitat protection for caribou varies considerably across jurisdictions, but is greatest within the Southern Mountain ecotype, where 22,000 km2 of remaining old forest have been protected from forest cutting in legal land reserves (47). This protection has resulted in 5 of 18 caribou ranges in this study having similar or higher levels of forest gain than forest loss (36) (SI Appendix, Table S1). In such areas, the degree of intensive population management needed to recover caribou is expected to diminish over time. However, in areas where habitat loss exceeds habitat recovery, intensive population treatments will have to be ongoing until there is a change in how natural resources are valued.
Methods
Our study included 18 caribou populations in Alberta, British Columbia, and Idaho, of which 12 were subjected to government-led management actions (hereafter referred to as treatments in an adaptive management context) and 6 were controls. We chose only 6 control populations to be conservative in matching ecological conditions as closely as possible to the treatment populations. However, almost all caribou populations in western Canada were rapidly declining; for example, during the same period, populations in Alberta were declining at a mean rate of −8% per year (48). The 12 treated populations in our study were subjected to four recovery actions; (i) predator reductions, (ii) prey reductions, (iii) translocation, and/or (iv) maternal penning (Fig. 2).
Although controversial in many conservation settings, there is a long history of predator (and prey) reduction to recover endangered species (34, 49), from removing feral goats (Capra spp.), to recover endangered island fauna (50), to removal of golden eagles on the Channel Islands, to recover the endangered Channel Island fox (25). Population reduction of wolves, however, is especially controversial given their heightened conservation status in the United States, and important trophic role (51). Nonetheless, wolves are nowhere near endangered or threatened in Canada and are widely distributed there, and conservative population estimates are >14,000 wolves in just Alberta and British Columbia (52). Field studies confirm that wolves are a leading cause of mortality and are the proximate cause of caribou declines (14, 22, 32, 53–56). Moreover, federal and provincial policies and legislation explicitly list predator and prey reduction as a required recovery action, along with habitat recovery, to recover endangered woodland caribou under Canada’s Species at Risk Act (37, 57, 58). Finally, predator removal was coordinated by provincial agencies usually via helicopter shooting [similar to the removal of feral goats on Galapagos, for example (50)] under the authority of the respective provincial wildlife Acts (59). Prey reductions were conducted through licensed hunting of moose by sport hunters, also through the authority of provincial wildlife acts and policies. Thus, despite the ethical issues surrounding removal of vertebrates (wolves, moose) to recover caribou (60), methods were permitted and enabled by federal and provincial legislation and policies. No university personnel were involved in planning or conducting predator reductions, thus obviating the need for university animal care review or approvals (see ref. 60). Similarly, caribou translocations in British Columbia were conducted exclusively by government staff supervised by the provincial wildlife veterinarian.
Caribou populations were monitored for responses to treatments between 2004 and 2018, whereas pretreatment monitoring dated back to 1994 (SI Appendix, Table S1). The 18 populations spanned four recognized caribou ecotypes: boreal, northern mountain, central mountain, and southern mountain (61). Boreal are classified by COSEWIC [Committee on the Status of Endangered Wildlife in Canada (62)] as threatened (n = 1 population); northern (n = 2), as of special concern; central (n = 6) and southern (n = 9), as endangered (61). Despite variation in their listed status, the bulk of our populations were endangered; thus, we use the term endangered to refer to the status of caribou throughout. Our response metric was the finite rate of population change (λ) (63) or, more specifically, the change in λ (Δλ) before and after treatments. There are two approaches to estimating λ of caribou populations depending on behavioral and habitat differences among ecotypes. The first approach is to estimate population growth rate using aerial surveys in areas where aerial sightability is high (64). In these cases, λ was calculated as λaerial = (Nt/N0)(1/t) (63). The second uses survival of radio-collared animals and population-level recruitment rates to estimate λ using a simple unstructured population model, the recruitment-mortality equation (65): λRM = S/(1 − R), where S is annual survival of adult females and R is recruitment.
For populations in British Columbia (n = 15), there are three ecotypes of woodland caribou (central, southern, and northern), and aerial survey methods differ slightly due to ecological differences. For the southern mountain ecotype (n = 9), survey estimates have been validated with 153 radio-collared animals. When snow depth exceeds 300 cm (3) in the upper subalpine, where the caribou dwell during late winter surveys, sightability is greater than 90%. Surveys were conducted only under such conditions, making population estimation straightforward. For the other six populations in British Columbia (central and northern ecotypes), mark-resight (54) with radio-marked caribou was used to correct population sizes, or all individuals were marked or identified through camera traps (66). Populations in Alberta (n = 3) are difficult to aerially survey because caribou live in dense coniferous forest, so population trend and associated uncertainty were estimated based on λRM (48), using the adjustment of ref. 67 to account for the delayed age at first reproduction of caribou. DeCesare et al. (67) showed that the λRM equation is algebraically identical to a Leftokvich stage matrix with three stages and thus provides identical results, but λRM is the convention used for monitoring woodland caribou. Although population estimates were not available in Alberta, minimum caribou observed indicated that all three populations had >50 animals at the start of treatments (57). Calibration and validation of the two approaches to estimating λ have been extensive (64, 67, 68). Serrouya et al. (64) compared λ for populations where both data sources (λaerial and λRM) were available, and found the correlation to be 0.78. This suggests that both metrics were comparable and that any biases within a population would be minimal over time because the same method (λaerial or λRM) was always used for each population. Additional details on the reliability of λ estimates presented in previously published studies can be found in the SI Appendix.
Like many ecosystem management cases (32), the intensity of treatments varied across areas. For example, neither prey nor predator reductions were ever 100%. In the SSE population, wolf removal occurred only on the Canadian portion of the range (Fig. 2). For the ALP population, treatment occurred on the winter range from 2007 to 2014 and then expanded to the winter and summer range from 2015 to 2017 (SI Appendix, Table S1). To index the intensity of treatment, we reported the number of wolves per 1,000 km2 removed per year; for moose, we reported the percentage reduction from the peak population size. The CON population also had a maternal penning trial that began in 2014, although this was a pilot study that was designed not to affect λ but to test the concept on a low number of animals (<20% of females). To isolate the effect of the moose reduction treatment, and to avoid a confound caused by maternal penning for caribou, comparisons in the Revelstoke (REV) study area (SI Appendix, Table S1) were ended in 2013 for the treated populations—CON, Columbia South (COS), Frisby-Queest (FBQ)—and the adjacent control populations (WGS and GRH). Isolating the effect of the moose reduction was important because this recovery tool had not been used before (30) in the context of apparent competition (unlike wolf reductions, which have been applied more frequently in this and other studies). Similarly, localized winter feeding of caribou occurred in the Kennedy Siding (KSI) population from 2014 to 2018, but was not formally considered a treatment. Results indicated no effect on λ, but some improvement to body condition was noted (66).
It was not just treatments that varied between populations, as the ultimate cause of population declines is habitat alteration (37, 58). We used an index of habitat alteration from remotely sensed forest loss data derived from Landsat (36) to control for the ultimate driver of caribou population trends: habitat alteration. The covariate was the proportion disturbed (early seral forest caused primarily by logging or petroleum development; ref. 36) within a population range, which was converted using the logit link. The proportion of early seral forest was included to test the hypothesis that less altered areas were more likely to have increased λ as a result of a treatment. Previous analyses showed that more early seral forests predicted lower caribou recruitment, as revealed in a national meta-analysis spanning 35 populations in the federal recovery strategy (37) and supported by theory and empirical studies across Canada. To contextualize the length of time that population treatments would be required, habitat alteration was also stratified by forest loss and forest gain based on the definition of ref. 36.
We conducted an ANCOVA to test our hypotheses by explaining Δλ as a result of recovery treatments and the proportion disturbed in each caribou range, with nontreatment (control) populations set as the intercept. For statistical analyses, λ was converted to the instantaneous rate of increase (r), λ = er (63), because r is centered on 0 and normally distributed. The dependent variable was the log response ratio, Δr, defined as ln (λafter) − ln (λbefore)—that is, the difference in population growth rates before vs. after treatments. Population size and treatment intensity were estimated quantitatively as described earlier, but were treated as qualitative factors for three reasons: (i) limited degrees of freedom are inherent in large-scale studies, (ii) population size was not available for the three herds in Alberta, and (iii) we did not have a common currency among treatment types to quantify intensity. All statistics were performed in R using the base lm package (69).
Supplementary Material
Acknowledgments
C. Gray, M. Dickie, and K. Benesh helped with data extraction and GIS analyses; and L. DeGroot conducted the SSE surveys. The West Moberly and Saulteau First Nations were instrumental in implementing treatments for KZA. Funding was provided by the Alberta and British Columbia provincial governments, Idaho Fish and Game, and Parks Canada for the caribou surveys we conducted. M.H. acknowledges funding from NASA through the Arctic Boreal Vulnerability Experiment (ABoVE) (Grant NNX15AW71A).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1816923116/-/DCSupplemental.
References
- 1.Caughley G. Directions in conservation biology. J Anim Ecol. 1994;63:215–244. [Google Scholar]
- 2.Carpenter SR. Ecological futures: Building an ecology of the long now. Ecology. 2002;83:2069–2083. [Google Scholar]
- 3.Doak DF, et al. Understanding and predicting ecological dynamics: Are major surprises inevitable? Ecology. 2008;89:952–961. doi: 10.1890/07-0965.1. [DOI] [PubMed] [Google Scholar]
- 4.Walters CJ, Holling CS. Large-scale management experiments and learning by doing. Ecology. 1990;71:2060–2068. [Google Scholar]
- 5.Fujitani M, McFall A, Randler C, Arlinghaus R. Participatory adaptive management leads to environmental learning outcomes extending beyond the sphere of science. Sci Adv. 2017;3:e1602516. doi: 10.1126/sciadv.1602516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCook LJ, et al. Adaptive management of the Great Barrier Reef: A globally significant demonstration of the benefits of networks of marine reserves. Proc Natl Acad Sci USA. 2010;107:18278–18285. doi: 10.1073/pnas.0909335107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westgate MJ, Likens GE, Lindenmayer DB. Adaptive management of biological systems: A review. Biol Conserv. 2013;158:128–139. [Google Scholar]
- 8.Johnson CN, et al. Biodiversity losses and conservation responses in the Anthropocene. Science. 2017;356:270–275. doi: 10.1126/science.aam9317. [DOI] [PubMed] [Google Scholar]
- 9.Hebblewhite M. Billion dollar boreal woodland caribou and the biodiversity impacts of the global oil and gas industry. Biol Conserv. 2017;206:102–111. [Google Scholar]
- 10.Bradshaw CJ, Warkentin IG, Sodhi NS. Urgent preservation of boreal carbon stocks and biodiversity. Trends Ecol Evol. 2009;24:541–548. doi: 10.1016/j.tree.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Hebblewhite M, White C, Musiani M. Revisiting extinction in national parks: Mountain caribou in Banff. Conserv Biol. 2010;24:341–344. doi: 10.1111/j.1523-1739.2009.01343.x. [DOI] [PubMed] [Google Scholar]
- 12.Festa-Bianchet M, Ray JC, Boutin S, Cote S, Gunn A. Conservation of caribou (Rangifer tarandus) in Canada: An uncertain future. Can J Zool. 2011;89:419–434. [Google Scholar]
- 13.Newmark WD. Extinction of mammal populations in western North American national parks. Conserv Biol. 1995;9:512–526. [Google Scholar]
- 14.Wittmer HU, Sinclair ARE, McLellan BN. The role of predation in the decline and extirpation of woodland caribou. Oecologia. 2005;144:257–267. doi: 10.1007/s00442-005-0055-y. [DOI] [PubMed] [Google Scholar]
- 15.Wittmer HU, McLellan BN, Serrouya R, Apps CD. Changes in landscape composition influence the decline of a threatened woodland caribou population. J Anim Ecol. 2007;76:568–579. doi: 10.1111/j.1365-2656.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 16.Shackleton D. Hoofed Mammals of British Columbia. University of British Columbia Press; Victoria, BC: 1999. [Google Scholar]
- 17.Haber GC. 1977. Socio-ecological dynamics of wolves and prey in a subarctic ecosystem. Ph.D dissertation (Univ. of British Columbia, Vancouver)
- 18.Rempel RS, Elkie PC, Rodgers AR, Gluck MJ. Timber-management and natural-disturbance effects on moose habitat: Landscape evaluation. J Wildl Manage. 1997;61:517–524. [Google Scholar]
- 19.Serrouya R, McLellan BN, Boutin S, Seip DR, Nielsen SE. Developing a population target for an overabundant ungulate for ecosystem restoration. J Appl Ecol. 2011;48:935–942. [Google Scholar]
- 20.Latham ADM, Latham MC, McCutchen NA, Boutin S. Invading white-tailed deer change wolf-caribou dynamics in northeastern Alberta. J Wildl Manage. 2011;75:204–212. [Google Scholar]
- 21.Fuller TK, Mech LD, Cochrane JF. Wolf population dynamics. In: Mech DL, Boitani L, editors. Wolves: Behavior, Ecology, and Conservation. University of Chicago Press; Chicago: 2003. pp. 161–191. [Google Scholar]
- 22.Seip DR. Factors limiting woodland caribou populations and their interrelationships with wolves and moose in southeastern British Columbia. Can J Zool. 1992;70:1494–1503. [Google Scholar]
- 23.Holt RD. Predation, apparent competition, and the structure of prey communities. Theor Popul Biol. 1977;12:197–29. doi: 10.1016/0040-5809(77)90042-9. [DOI] [PubMed] [Google Scholar]
- 24.DeCesare NJ, Hebblewhite M, Robinson HS, Musiani M. Endangered, apparently: The role of apparent competition in endangered species conservation. Anim Conserv. 2010;13:353–362. [Google Scholar]
- 25.Courchamp F, Woodroffe R, Roemer G. Removing protected populations to save endangered species. Science. 2003;302:1532. doi: 10.1126/science.1089492. [DOI] [PubMed] [Google Scholar]
- 26.Roemer GW, Donlan CJ, Courchamp F. Golden eagles, feral pigs, and insular carnivores: How exotic species turn native predators into prey. Proc Natl Acad Sci USA. 2002;99:791–796. doi: 10.1073/pnas.012422499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haddad NM, et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv. 2015;1:e1500052. doi: 10.1126/sciadv.1500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wittmer HU, Ahrens RNM, McLellan BN. Viability of mountain caribou in British Columbia, Canada: Effects of habitat change and population density. Biol Conserv. 2010;143:86–93. [Google Scholar]
- 29.Hervieux D, Hebblewhite M, Stepnisky D, Bacon M, Boutin S. Managing wolves (Canis lupus) to recover threatened woodland caribou (Rangifer tarandus caribou) in Alberta. Can J Zool. 2014;92:1029–1037. [Google Scholar]
- 30.Serrouya R, McLellan BN, van Oort H, Mowat G, Boutin S. Experimental moose reduction lowers wolf density and stops decline of endangered caribou. PeerJ. 2017;5:e3736. doi: 10.7717/peerj.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serrouya R, Wittmann MJ, McLellan BN, Wittmer HU, Boutin S. Using predator-prey theory to predict outcomes of broadscale experiments to reduce apparent competition. Am Nat. 2015;185:665–679. doi: 10.1086/680510. [DOI] [PubMed] [Google Scholar]
- 32.Hayes RD, et al. Experimental reduction of wolves in the Yukon: Ungulate responses and management implications. Wildl Monogr. 2003;152:1–35. [Google Scholar]
- 33.Smith RK, Pullin AS, Stewart GB, Sutherland WJ. Effectiveness of predator removal for enhancing bird populations. Conserv Biol. 2010;24:820–829. doi: 10.1111/j.1523-1739.2009.01421.x. [DOI] [PubMed] [Google Scholar]
- 34.Jones HP, et al. Invasive mammal eradication on islands results in substantial conservation gains. Proc Natl Acad Sci USA. 2016;113:4033–4038. doi: 10.1073/pnas.1521179113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orians G, et al. Wolves, Bears, and Their Prey in Alaska. National Academy Press; Washington, DC: 1997. [Google Scholar]
- 36.Hansen MC, et al. High-resolution global maps of 21st-century forest cover change. Science. 2013;342:850–853. doi: 10.1126/science.1244693. [DOI] [PubMed] [Google Scholar]
- 37.Environment Canada 2012. Recovery Strategy for the Woodland Caribou (Rangifer tarandus caribou), Boreal Population, in Canada, Species at Risk Act Recovery Strategy Series (Environment Canada, Ottawa), p xi, 138 p.
- 38.Holt RD, Bonsall MB. Apparent competition. Annu Rev Ecol Evol Syst. 2017;48:447–471. [Google Scholar]
- 39.Serrouya R, McLellan BN, Boutin S. Testing predator-prey theory using broad-scale manipulations and independent validation. J Anim Ecol. 2015;84:1600–1609. doi: 10.1111/1365-2656.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pérez I, et al. What is wrong with current translocations? A review and a decision-making proposal. Front Ecol Environ. 2012;10:494–501. [Google Scholar]
- 41.Leech H, Jelinski DE, DeGroot L, Kuzyk G. The temporal niche and seasonal differences in predation risk to translocated and resident woodland caribou (Rangifer tarandus caribou) Can J Zool. 2017;95:809–820. [Google Scholar]
- 42.Wittmer HU, Elbroch LM, Marshall AJ. Good intentions gone wrong: Did conservation management threaten endangered huemul deer Hippocamelus bisulcus in the future Patagonia National Park? Oryx. 2013;47:393–402. [Google Scholar]
- 43.Compton BB, Zager P, Servheen G. Survival and mortality of translocated woodland caribou. Wildl Soc Bull. 1995;23:490–496. [Google Scholar]
- 44.Krebs CJ. 2009. Ecology: The Experimental Analysis of Distribution and Abundance (Benjamin Cummings, San Francisco), 6th Ed, p xvi, 655 p.
- 45.Desrosier F. It Takes a Village. FD Productions; Revelstoke, BC: 2018. [Google Scholar]
- 46.Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333:1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- 47.British Columbia Ministry of Environment, Species at Risk Coordination, Victoria 2009. A review of management actions to recover mountain caribou in British Columbia (BCMOE, Species at Risk Coordination, Victoria, BC), Number 2913682.
- 48.Hervieux D, et al. Widespread declines in woodland caribou (Rangifer tarandus caribou) continue in Alberta. Can J Zool. 2013;91:872–882. [Google Scholar]
- 49.Goodrich J, Buskirk S. Control of abundant native vertebrates for conservation of endangered species. Conserv Biol. 1995;9:1357–1364. [Google Scholar]
- 50.Campbell K, Donlan CJ. Feral goat eradications on islands. Conserv Biol. 2005;19:1362–1374. [Google Scholar]
- 51.Ripple WJ, et al. Status and ecological effects of the world’s largest carnivores. Science. 2014;343:1241484. doi: 10.1126/science.1241484. [DOI] [PubMed] [Google Scholar]
- 52.Kuzyk GW, Hatter IW. Using ungulate biomass to estimate abundance of wolves in British Columbia. Wildl Soc Bull. 2014;38:878–883. [Google Scholar]
- 53.Whittington J, et al. Caribou encounters with wolves increase near roads and trails: A time‐to‐event approach. J Appl Ecol. 2011;48:1535–1542. [Google Scholar]
- 54.Wittmer HU, et al. Population dynamics of the endangered mountain ecotype of woodland caribou (Rangifer tarandus caribou) in British Columbia, Canada. Can J Zool. 2005;83:407–418. [Google Scholar]
- 55.Edmonds EJ. Population status, distribution, and movements of woodland caribou in west central Alberta. Can J Zool. 1988;66:817–826. [Google Scholar]
- 56.Rettie WJ, Messier F. Dynamics of woodland caribou populations at the southern limit of their range in Saskatchewan. Can J Zool. 1998;76:251–259. [Google Scholar]
- 57.Environmental Services Association of Alberta . 2017. Draft provincial caribou range plan (ESAA, Edmonton, AB) [Google Scholar]
- 58.Environment Canada . Species at Risk Act Recovery Strategy Series. Environment Canada; Ottawa: 2014. Recovery strategy for the woodland caribou, southern mountain population (Rangier tarandus caribou) in Canada. [Google Scholar]
- 59.Alberta Go. In Revised Statutes of Alberta 2000, Chapter W-10. Government of Alberta; Edmonton, AB: 2006. Wildlife act. [Google Scholar]
- 60.Hervieux D, Hebblewhite M, Stepnisky D, Bacon M, Boutin S. Addendum to “Managing wolves (Canis lupus) to recover threatened woodland caribou (Rangifer tarandus caribou) in Alberta”. Can J Zool. 2015;93:245–247. [Google Scholar]
- 61.Ray JC, et al. Conservation status of caribou in the western mountains of Canada: Protections under the Species at Risk Act, 2002–2014. Rangifer. 2015;35:49–80. [Google Scholar]
- 62.Committee on the Status of Endangered Wildlife in Canada 2011. Designatable units for caribou (Rangifer tarandus) in Canada (COSEWIC, Ottawa)
- 63.Caughley G. Analysis of Vertebrate Populations. Wiley; New York: 1977. [Google Scholar]
- 64.Serrouya R, et al. Comparing population growth rates between census and recruitment-mortality models. J Wildl Manage. 2017;81:297–305. [Google Scholar]
- 65.Hatter IW, Bergerud WA. Moose recruitment, adult mortality and rate of change. Alces. 1991;27:65–73. [Google Scholar]
- 66.Seip D, Jones E. 2018. Population status of Central Mountain caribou herds in British Columbia and response to recovery management actions, 2018 (British Columbia Ministry of Environment, Pringe George, BC)
- 67.DeCesare NJ, et al. Estimating ungulate recruitment and growth rates using age ratios. J Wildl Manage. 2012;76:144–153. [Google Scholar]
- 68.DeCesare NJ, Hebblewhite M, Lukacs PM, Hervieux D. Evaluating sources of censoring and truncation in telemetry‐based survival data. J Wildl Manage. 2016;80:138–148. [Google Scholar]
- 69.R Core Team 2018. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.