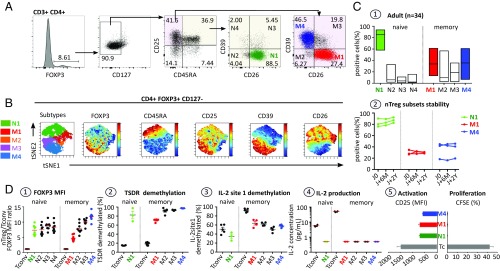
Fig. 1.
FOXP3 nTreg heterogeneity in healthy human PBMCs. (A) Flow cytometry gating strategy for identifying the major nTreg subsets in human PBMCs based on FOXP3, CD127, CD25, CD45RA, CD26, and CD39 markers. (B) After initial gating on the CD3+CD4+FOXP3+CD127− nTreg population, the gated cells were clustered using viSNE (Cytobank). Cells are color-coded according to the expression level of FOXP3, CD45RA, CD25, CD39, and CD26 markers. (C1) Boxplots illustrating the distribution of the four nTreg subsets based on the expression of CD39 and CD26 in naive and memory nTreg compartments. (C2) Longitudinal analysis of nTreg subset frequencies in three individuals for a >2-y period. (D) Phenotypic, epigenetic, and physiological characteristics of FACS-sorted nTreg subsets. (D1) Summary plot of the MFI ratio of FOXP3 expression on Treg subsets to Tconvs. (D2 and D3) Scatterplot indicating FOXP3-TSDR (D2) and IL-2 CpG site 1 demethylation status (D3) of the five major FACS-sorted nTreg subsets (N1, M1–M4) and the two conventional T cells (naive and memory) as assessed by bisulfite pyrosequencing. Carboxyfluorescein succinimidyl ester (CFSE)-labeled nTreg subsets (N1, M1, and M4) and Tconvs (4 × 104 per well) were stimulated with a low dose of plate-bound anti-CD3 mAb (pbαCD3; 0.5 µg/mL) in the presence of irradiated feeder. (D4) IL-2 concentration in culture supernatant from 40 h-stimulated Tconv cells and nTreg subsets as measured by ELISA. (D5) T cell activation status and T cell proliferation were evaluated by the MFI of CD25 and the CFSE dilution assay, respectively. Data are expressed as mean ± SEM.