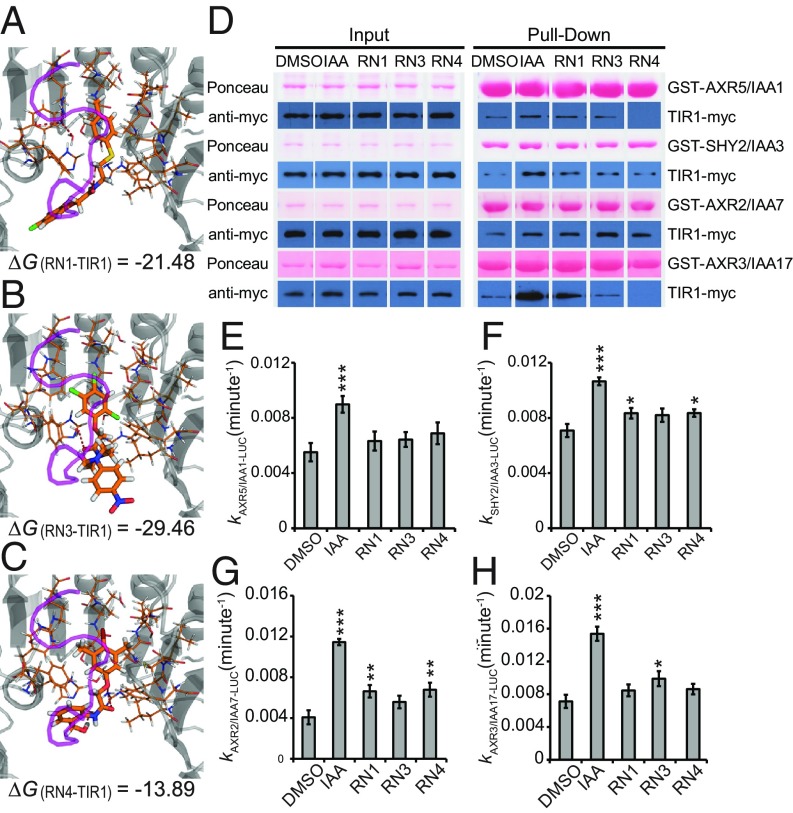
Fig. 3.
RN3 and RN4 act as selective agonists of auxin. (A–C) The RNs showed different thermodynamic stabilities from the calculated free energies (ΔG). RN1 (A), RN3 (B), and RN4 (C) were sterically favorable for the binding of the AUX/IAA7 DII degron. TIR1 is presented in gray and the AUX/IAA7 DII degron, which was included afterward to observe any conflict with the RNs, is in purple. Thermodynamic stability was computed within the TIR1 auxin binding pocket and the most stable conformations are represented. (D) The potential of the RNs (at 50 μM) to promote the formation of the coreceptor complex was performed using in vitro translated TIR1-myc and recombinant GST-AUX/IAAs. Depending on the GST-AUX/IAA translational fusion used for the in vitro GST pull-down, the RNs selectively increased the recovery of TIR1-myc. (E–H) AUX/IAA degradation was assayed in planta using Arabidopsis lines constitutively expressing different AUX/IAA-LUCs in the presence of RNs at 50 μM. Effects of the RNs on the in vivo degradation rate k of AXR5/IAA1-LUC (E), SHY2/IAA3-LUC (F), AXR2/IAA7-LUC (G), or AXR3/IAA17-LUC (H) translational fusions. Statistical analyses were performed using the Student’s t test. Means ± SEM are shown, n = 30 seedlings across five independent replicates, *P < 0.05, **P < 0.01, ***P < 0.001.