Abstract
We evaluated the effectiveness of ledipasvir/sofosbuvir (LDV/SOF) in treating hepatitis C virus (HCV) genotype 1 and SOF/velpatasvir (SOF/VEL) for all genotypes among people who inject drugs (PWID) and those not injecting drugs and who were on or off opioid agonist therapy (OAT). Study participants comprised a population‐based cohort in British Columbia, Canada. The British Columbia Hepatitis Testers Cohort includes data on individuals tested for HCV from 1990 to 2016 that are integrated with medical visits, hospitalization, and prescription drug data. We classified study participants as off OAT/recent injection drug use (off‐OAT/RIDU), off OAT/past IDU (off‐OAT/PIDU), off OAT/no IDU (off‐OAT/NIDU), on OAT/IDU (on‐OAT/IDU), and on OAT/no IDU (on‐OAT/NIDU). We assessed sustained virologic response (SVR) 10 weeks after HCV treatment among study groups treated with LDV/SOF or SOF/VEL until January 13, 2018. Analysis included 5,283 eligible participants: 390 off‐OAT/RIDU, 598 off‐OAT/PIDU, 3,515 off‐OAT/NIDU, 609 on‐OAT/IDU, and 171 on‐OAT/NIDU. The majority were male patients (64%‐74%) and aged ≥50 years (58%‐85%). The SVRs for off‐OAT/RIDU, off‐OAT/PIDU, off‐OAT/NIDU, on‐OAT/IDU, and on‐OAT/NIDU were 91% (355/390), 95% (570/598), 96% (3,360/3,515), 93% (567/609), and 95% (163/171), respectively. Among those with no SVR, 14 individuals died while on treatment or before SVR assessment, including 4 from illicit drug overdose. In the overall multivariable model, off‐OAT/RIDU, on‐OAT/IDU, male sex, cirrhosis, treatment duration <8 weeks, treatment duration 8 weeks, and treatment with SOF/VEL were associated with not achieving SVR. Conclusion: In this large real‐world cohort, PWID and/or those on OAT achieved high SVRs, although slightly lower than people not injecting drugs. This finding also highlights the need for additional measures to prevent loss to follow‐up and overdose‐related deaths among PWID.
Abbreviations
- AOR
adjusted odds ratio
- BCCDC‐PHL
British Columbia Centre for Disease Control Public Health Laboratory
- BC‐HTC
British Columbia Hepatitis Testers Cohort
- CI
confidence interval
- DAA
direct‐acting antiviral
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IDU
injection drug use
- LDV/SOF
ledipasvir/sofosbuvir
- OAT
opioid agonist therapy
- off‐OAT/NIDU
off opioid agonist therapy/no injection drug use
- off‐OAT/PIDU
off opioid agonist therapy/past injection drug use
- off‐OAT/RIDU
off opioid agonist therapy/recent injection drug use
- on‐OAT/IDU
on opioid agonist therapy/injection drug use
- on‐OAT/NIDU
on opioid agonist therapy/no injection drug use
- PWID
people who inject drugs
- Q
quartile
- RBV
ribavirin
- SOF/VEL
sofosbuvir/velpatasvir
- SVR
sustained virologic response
Hepatitis C virus (HCV) infection is a global concern, with more than 70 million people infected.1 Most new infections and a large proportion of prevalent HCV infections in developed countries occur in people who inject drugs (PWID). The prevalence of HCV among PWID ranges from 50% to 80% in various regions of the world.2 In British Columbia, Canada, approximately 30% of individuals with prevalent infections and 70% to 80% of those with acute infections have a history of injection drug use (IDU).3
Despite a very high disease burden and availability of curative treatments since the early 2000s, PWID were less likely to receive treatment during the interferon‐based treatment era compared to other population groups.4 Major reasons for low treatment uptake included concerns related to tolerability, poor compliance, and reinfection.5 Highly effective, short course, and well‐tolerated direct‐acting antiviral (DAA) agents are a major medical breakthrough that have mitigated some of these concerns for groups previously impeded from optimal treatment access, such as PWID, people living with human immunodeficiency virus (HIV), and those with advanced‐stage liver disease. However, there is an urgent need to evaluate whether gaps in treatment access remain for PWID.6, 7 Recent data from randomized controlled trials (RCTs) assessing the efficacy of grazoprevir/elbasvir and sofosbuvir/velpatasvir (SOF/VEL) among PWID on opioid agonist therapy (OAT) and participants in other trials on OAT showed a high efficacy of various DAA regimens comparable to no IDU among HCV‐infected population groups.8, 9, 10 However, PWID participating in RCTs are very different from those in a real‐world situation, and concerns remain about compliance, loss to follow‐up, and treatment effectiveness.11 Although data on the treatment of PWID from real‐world settings are emerging, many of these studies are small and enroll patients from single practice or community programs, with the exception of a recent German study.12, 13, 14, 15, 16 As such, broader population‐based data on the treatment of PWID with DAAs are limited. Such data are critical to informing treatment effectiveness and expanding treatment access to this marginalized group and for reaching the World Health Organization’s goals of HCV elimination.
We evaluated the effectiveness of ledipasvir/SOF (LDV/SOF) in treating HCV genotype 1 and SOF/VEL for all genotypes among PWID and those not injecting drugs who were on or off OAT in a real‐world clinical practice.
Participants and Methods
The Cohort
For this analysis, we used data from the British Columbia Hepatitis Testers Cohort (BC‐HTC). Details of the cohort creation and epidemiologic characteristics have been reported.3, 17 The BC‐HTC includes all individuals tested for HCV or HIV or reported as a case of hepatitis B virus (HBV), HCV, HIV, or active tuberculosis in British Columbia between 1990 and 2016 (Supporting Table S1). These data are integrated with data on medical visits, hospitalizations, cancers, prescription drugs, and deaths. All residents in British Columbia are registered in the publicly funded insurance plan that acts as a single‐payer system and covers services provided by fee‐for‐service practitioners. HCV laboratory testing for the entire province is performed at the British Columbia Centre for Disease Control Public Health Laboratory (BCCDC‐PHL), except for <5% of screening tests performed at a regional laboratory that sends positive tests to BCCDC‐PHL for confirmation and HCV RNA testing. All dispensed prescriptions in the province, including HCV treatments and OAT, are recorded in a central system called PharmaNet.
Study Population and HCV Treatments
This analysis included individuals who were HCV positive, who initiated treatment with SOF‐based regimens LDV/SOF or SOF/VEL until January 13, 2018, and who were followed for treatment response until October 9, 2018. In British Columbia, LDV/SOF became available on October 14, 2014, and SOF/VEL became available on July 14, 2016. LDV/SOF, with or without ribavirin (RBV), was used for treating people with genotype 1 for 8, 12, or 24 weeks, based on prior treatment experience, fibrosis level, and viral load. SOF/VEL with or without RBV was prescribed for treatment of all genotypes for 12 weeks. Treatment in British Columbia during this study period was provided by hepatologists, infectious disease specialists, and some general practitioners. Addiction services and HCV treatment are co‐located in some clinics. For this analysis, all participants taking LDV/SOF were followed for at least 36 weeks from treatment initiation, and those taking SOF/VEL were followed for 24 weeks to allow for treatment response assessment. Treatment duration was classified as 8, 12, and 24 weeks. We excluded those with no RNA after treatment initiation or those with negative RNA tests between the end of treatment and week 10 after treatment but no RNA test following week 10 after treatment to assess sustained virologic response (SVR).
The study population was classified into those who were on OAT and not on OAT. OAT in British Columbia includes methadone and buprenorphine/naloxone for maintenance treatment.18 Most OAT dispensations are directly witnessed, but a few individuals receive 2 to 3 days of take‐home doses. Individuals receiving OAT at the time of HCV treatment initiation (28 days before treatment and on OAT while on treatment) were considered to be on OAT. Within on and off OAT groups, we further classified study participants as recent PWID, former PWID, and those not injecting drugs. PWID were identified based on an algorithm using fee‐for‐service, procedure, and/or diagnostic codes for medical visits, hospitalization, or prescription dispensation and validated in the BC‐HTC subset using interview‐based risk factor data.19 For this analysis, we used the IDU algorithm with optimal sensitivity (78%) and specificity (83%); this required two medical visits and one hospitalization.19 Individuals with drug‐related diagnoses in the 3 years prior to treatment were considered recent PWID, and those with diagnoses more than 3 years prior were classified as former PWID (Supporting Table S1).
Assessment of SVR
SVR was defined as an undetectable HCV RNA 10 weeks after treatment conclusion. As in other studies, a 10‐week time period instead of 12 weeks was chosen to account for variability in testing in clinical practice.20 A small proportion of individuals were tested at weeks 10 and 11. Patients were categorized as not achieving SVR if they had detectable HCV RNA after the end of treatment, had no viral load testing after the end of treatment, or had detectable HCV RNA on their last HCV viral load test while on treatment or within 10 weeks of the treatment end. To be more conservative in SVR assessment, we excluded patients with undetectable HCV RNA on their last test, either while on treatment or after treatment ended, but with no test at or after 10 weeks of treatment end. Those who never had a negative test on or after treatment were considered as nonresponsive, whereas those who had at least one negative RNA test on or after treatment followed by a positive test for SVR assessment were considered relapsed. Plasma HCV RNA levels were determined using the Abbott RealTime HCV assay (Abbott Molecular, Inc., Mississauga, Canada) with a lower limit of detection for HCV RNA of 12 IU/mL.
Assessment of Covariates
Demographic characteristics included sex, age, birth cohort, and social and material deprivation quintiles.21 Assessment of diabetes, major mental illness, cirrhosis, decompensated cirrhosis, problematic alcohol use, and a composite of 30 comorbidities as the Elixhauser comorbidity index was based on algorithms derived from medical visits, hospitalization, or prescription dispensation data using fee‐for‐service, procedure, and/or diagnostic codes.22, 23, 24, 25 (Supporting Table S1)
Analyses
We described characteristics of those on and off OAT by PWID status. We computed overall SVR and compared SVR by participant characteristics among OAT/PWID groups using Pearson’s chi‐square test or Fisher’s exact test, as appropriate. For those on OAT, we combined recent and former PWID for optimal sample size. We performed multivariable logistic regression analysis to compute adjusted odds ratios (AORs) and their 95% confidence intervals (CIs) for not achieving SVR to identify predictors of treatment response. All analyses were conducted in SAS/STAT software version 9.4, and all tests were two‐sided at a significance level of 0.05.
This study was approved by the University of British Columbia Research Ethics Board (H14‐01649).
Results
Overall, 6,678 participants initiated treatment with LDV/SOF or SOF/VEL with or without RBV. Of 5,950 with adequate follow‐up, 667 were excluded for lack of any HCV RNA test, no HCV RNA test after treatment initiation, or a negative HCV RNA test either during or between the end of treatment and week 10 after treatment but lack of an RNA test after 10 weeks to determine SVR. Of 392 individuals in this last group, 54 (14%) died during treatment or before week 10 after the end of treatment; 9 died from illicit drug‐related causes before assessment of SVR, and an additional person died at week 17 after treatment. Of the 5,283 eligible participants (evaluable population), 3,413 and 1,574 were treated with LDV/SOF and SOF/VEL, respectively (Fig. 1). Among these, there were 390 individuals off OAT and recent IDU (off‐OAT/RIDU), 598 off OAT and past IDU (off‐OAT/PIDU), 3,515 off OAT and no IDU (off‐OAT/NIDU), 609 on OAT with IDU (on‐OAT/IDU), and 171 on OAT with no IDU (on‐OAT/NIDU).
Figure 1.
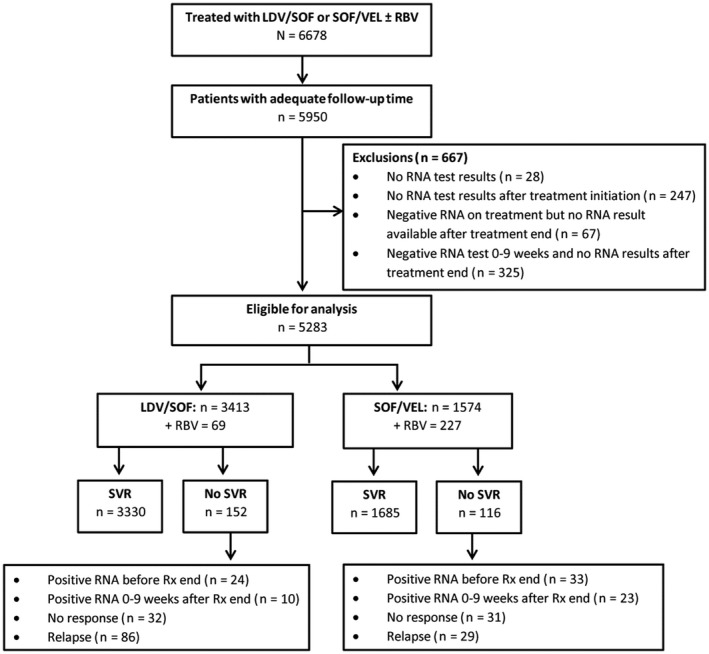
Flow of participants who initiated HCV treatment.
Participant Profile
In all treatment groups, the majority were male patients (64%‐74%), aged 50 to 59 years (40%‐50%), and in the 1945‐1964 birth cohort (51%‐81%), which was highest in the off‐OAT/NIDU group (81%) (Table 1). PWID (off‐OAT/RIDU, off OAT/PIDU, and on‐OAT/IDU) in comparison to off‐OAT/NIDU had a higher proportion with HIV (23%, 15%, and 27% versus 5%, respectively) or HBV coinfection (13%, 12%, and 14% versus 5%), problematic alcohol use (65%, 55%, and 51% versus 14%), or major mental illness (66%, 61%, and 59% versus 18%). A higher proportion of those in the off‐OAT/NIDU group had previous treatment compared to the off‐OAT/RIDU group (22% versus 15%, respectively). A higher proportion of off‐OAT/RIDU received SOF/VEL compared to the off‐OAT/NIDU group (41% versus 31%, respectively) (Table 1).
Table 1.
Characteristics of PWID and/or are on OAT at Treatment Initiation, British Columbia Hepatitis Testers Cohort
| Covariates | Off‐OAT/RIDU | Off‐OAT/PIDU | Off‐OAT/NIDU | On‐OAT/IDU | On‐OAT/NIDU |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| n | 390 | 598 | 3,515 | 609 | 171 |
| Birth cohort | |||||
| <1945 | 10 (1.7) | 207 (5.9) | 2 (0.3) | 1 (0.6) | |
| 1945‐1964 | 236 (60.5) | 419 (70.1) | 2,833 (80.6) | 313 (51.4) | 102 (59.6) |
| 1965‐1974 | 97 (24.9) | 130 (21.8) | 315 (9) | 170 (27.9) | 40 (23.4) |
| ≥1975 | 57 (14.6) | 39 (6.5) | 160 (4.6) | 124 (20.3) | 28 (16.4) |
| Age | |||||
| <50 | 131 (33.6) | 142 (23.8) | 366 (10.4) | 247 (40.6) | 57 (33.3) |
| 50‐59 | 179 (45.9) | 293 (49) | 1,403 (39.9) | 273 (44.9) | 85 (49.8) |
| ≥60 | 80 (20.5) | 163 (27.2) | 1,746 (49.7) | 89 (14.6) | 29 (17) |
| Sex | |||||
| Female | 117 (30) | 213 (35.6) | 1,167 (33.2) | 219 (35.9) | 45 (26.3) |
| Male | 273 (70) | 385 (64.3) | 2,348 (66.8) | 390 (64.1) | 126 (73.7) |
| Ethnicity | |||||
| White | 376 (96.4) | 579 (96.8) | 3,202 (91.1) | 596 (97.9) | 158 (92.4) |
| Others | 14 (3.6) | 19 (3.2) | 313 (8.9) | 13 (2.1) | 13 (7.6) |
| Treatment duration | |||||
| <8 weeks | 4 (1.1) | 5 (0.9) | 12 (0.3) | 6 (1) | |
| 8 weeks | 74 (19) | 84 (14.1) | 695 (19.8) | 93 (15.3) | 26 (15.2) |
| 12 weeks | 267 (68.4) | 430 (71.9) | 2,265 (64.4) | 446 (73.2) | 127 (74.3) |
| >12‐<24 weeks | 10 (2.6) | 4 (0.7) | 54 (1.5) | 9 (1.4) | 1 (0.6) |
| 24 weeks | 35 (9) | 75 (12.5) | 489 (13.9) | 55 (9) | 17 (9.9) |
| Previous treatment | |||||
| No | 333 (85.3) | 470 (78.6) | 2,762 (78.6) | 524 (86) | 150 (87.7) |
| Yes | 57 (14.6) | 128 (21.4) | 753 (21.4) | 85 (14) | 21 (12.3) |
| HCV RNA viral load (IU/mL)* | |||||
| <124,677 (Q1) | 98 (25.1) | 160 (26.7) | 850 (24.1) | 164 (26.9) | 43 (25.2) |
| 124,677‐670,049 (Q2) | 114 (29.2) | 126 (21.1) | 866 (24.6) | 160 (26.3) | 48 (28.1) |
| 670,049‐2,212,170 (Q3) | 81 (20.8) | 149 (24.9) | 920 (26.1) | 122 (20) | 44 (25.8) |
| ≥2,212,170 (Q4) | 97 (24.9) | 158 (26.4) | 860 (24.5) | 163 (26.8) | 36 (21.1) |
| Missing | 5 (0.8) | 19 (0.5) | |||
| Diabetes* | |||||
| No | 347 (89) | 541 (90.5) | 3,179 (90.4) | 557 (91.5) | 169 (98.9) |
| Yes | 43 (11) | 57 (9.5) | 336 (9.6) | 52 (8.5) | 2 (1.2) |
| Cirrhosis* | |||||
| No | 351 (90) | 533 (89.1) | 3,137 (89.2) | 567 (93.1) | 165 (96.5) |
| Yes | 39 (10) | 65 (10.8) | 378 (10.8) | 42 (6.9) | 6 (3.5) |
| Decompensated cirrhosis | |||||
| No | 371 (95.2) | 564 (94.3) | 3,324 (94.5) | 580 (95.2) | 169 (98.9) |
| Yes | 19 (4.9) | 34 (5.7) | 191 (5.5) | 29 (4.8) | 2 (1.2) |
| HBV | |||||
| No | 338 (86.6) | 526 (87.9) | 3,351 (95.3) | 524 (86.1) | 160 (93.6) |
| Yes | 52 (13.3) | 72 (12.1) | 164 (4.7) | 85 (13.9) | 11 (6.4) |
| HIV | |||||
| No | 299 (76.6) | 507 (84.8) | 3,336 (94.9) | 446 (73.2) | 149 (87.1) |
| Yes | 91 (23.4) | 91 (15.2) | 179 (5.1) | 163 (26.8) | 22 (12.9) |
| Problematic alcohol use | |||||
| Recent | 154 (39.5) | 65 (10.8) | 151 (4.3) | 102 (16.7) | 8 (4.7) |
| Past | 98 (25.1) | 267 (44.7) | 339 (9.6) | 211 (34.7) | 28 (16.4) |
| None | 138 (35.4) | 266 (44.5) | 3,025 (86.1) | 296 (48.6) | 135 (79) |
| Mental illness | |||||
| No | 132 (33.8) | 232 (38.8) | 2,872 (81.8) | 248 (40.8) | 136 (79.6) |
| Yes | 258 (66.1) | 366 (61.2) | 643 (18.3) | 361 (59.2) | 35 (20.5) |
| Elixhauser comorbidity index | |||||
| No (0) | 46 (11.8) | 49 (8.2) | 1,705 (48.5) | 71 (11.7) | 119 (69.6) |
| Yes (≥1) | 344 (88.2) | 549 (91.8) | 1,810 (51.5) | 538 (88.3) | 52 (30.4) |
| Material deprivation | |||||
| Q1 (most privileged) | 70 (17.9) | 80 (13.3) | 513 (14.6) | 120 (19.7) | 25 (14.6) |
| Q2 | 45 (11.6) | 84 (14.1) | 667 (18.9) | 77 (12.6) | 21 (12.3) |
| Q3 | 56 (14.4) | 106 (17.7) | 760 (21.7) | 97 (15.9) | 31 (18.1) |
| Q4 | 91 (23.4) | 159 (26.6) | 779 (22.1) | 108 (17.8) | 43 (25.2) |
| Q5 (most deprived) | 127 (32.5) | 164 (27.4) | 760 (21.6) | 192 (31.6) | 48 (28) |
| Unknown | 1 (0.3) | 5 (0.8) | 36 (1) | 15 (2.5) | 3 (1.8) |
| Social deprivation | |||||
| Q1 (most privileged) | 25 (6.4) | 54 (9) | 400 (11.4) | 39 (6.4) | 23 (13.5) |
| Q2 | 37 (9.5) | 70 (11.7) | 473 (13.4) | 58 (9.5) | 12 (7) |
| Q3 | 34 (8.8) | 92 (15.4) | 698 (19.9) | 71 (11.6) | 23 (13.5) |
| Q4 | 71 (18.2) | 133 (22.2) | 845 (24.1) | 94 (15.4) | 34 (19.9) |
| Q5 (most deprived) | 222 (57) | 244 (40.8) | 1,063 (30.2) | 332 (54.5) | 76 (44.4) |
| Unknown | 1 (0.3) | 5 (0.8) | 36 (1) | 15 (2.5) | 3 (1.8) |
| Treatment year | |||||
| 2010‐2014 | 5 (1.3) | 6 (1) | 76 (2.2) | 4 (0.7) | |
| 2015‐2017 | 385 (98.7) | 592 (99) | 3,439 (97.8) | 605 (99.3) | 171 (100) |
| Treatment type | |||||
| LDV/SOF | 227 (58.2) | 375 (62.7) | 2,368 (67.4) | 351 (57.7) | 92 (53.8) |
| LDV/SOF + RBV | 3 (0.8) | 6 (1) | 52 (1.5) | 4 (0.7) | 4 (2.3) |
| SOF/VEL | 145 (37.2) | 185 (30.9) | 959 (27.3) | 217 (35.7) | 68 (39.7) |
| SOF/VEL + RBV | 15 (3.9) | 32 (5.3) | 136 (3.9) | 37 (6.1) | 7 (4.1) |
| Genotype | |||||
| Genotype 1 | 267 (68.5) | 447 (74.7) | 2,710 (77.1) | 427 (70.1) | 122 (71.3) |
| Genotype 2 | 20 (5.2) | 39 (6.6) | 265 (7.6) | 23 (3.8) | 4 (2.4) |
| Genotype 3 | 91 (23.3) | 92 (15.4) | 371 (10.6) | 135 (22.2) | 41 (24) |
| Other/unknown | 12 (3.1) | 20 (3.4) | 169 (4.8) | 24 (4) | 4 (2.3) |
Assessed at the last treatment prescription.
Abbreviation: Q, quartile.
Response to HCV Treatment
SVR was achieved in 91% (355/390) of the off‐OAT/RIDU group, 95% (570/598) in the off‐OAT/PIDU group, 96% (3,360/3,515) in the off‐OAT/NIDU group, 93% (567/609) in the on‐OAT/IDU group, and 95% (163/171) in the on‐OAT/NIDU group (Table 2). A higher proportion of those receiving LDV/SOF achieved SVR compared to the SOF/VEL group (96% [3,330/3,482]) versus (94% [1,685/1,801], respectively; P = 0.001). Among those who did not achieve SVR with LDV/SOF (152), 34 (22%) could not be assessed for SVR (lost to follow‐up), 32 (21%) did not respond to treatment, and 86 (57%) relapsed after an initial response. Within the LDV/SOF group, 38% (13/34) of loss to follow‐up was related to death. Of those who did not achieve SVR with SOF/VEL, 56 (48%) could not be assessed for SVR (lost to follow‐up), 31 (27%) did not respond to treatment, and 29 (25%) relapsed after an initial response.
Table 2.
SVR Among Patients Treated with LDV/SOF and SOF/VEL for Genotype 1 by IDU and OAT Status, British Columbia Hepatitis Testers Cohort
| Covariates | Off‐OAT/RIDU | Off‐OAT/PIDU | Off‐OAT/NIDU | On‐OAT/IDU | On‐OAT/NIDU | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SVR | No SVR | % SVR | SVR | No SVR | SVR | No SVR | % SVR | SVR | No SVR | SVR | No SVR | % SVR | SVR | No SVR | |
| n (%) | 355 (91) | 35 (9) | 570 (95) | 28 (5) | 3,360 (96) | 155 (4) | 567 (93) | 42 (7) | 163 (95) | 8 (5) | |||||
| Birth cohort | |||||||||||||||
| <1945 | 0 | 0 | 0 | 10 (1.8) | 0 (0) | 100 | 189 (5.6) | 18 (11.6) | 91.3 | 2 (0.4) | 0 (0) | 100 | 1 (0.6) | 0 (0) | 100 |
| 1945‐1964 | 216 (60.8) | 20 (57.1) | 91.5 | 400 (70.2) | 19 (67.9) | 95.5 | 2,716 (80.8) | 117 (75.5) | 95.9 | 298 (52.6) | 15 (35.7) | 95.2 | 98 (60.1) | 4 (50) | 96.1 |
| 1965‐1974 | 85 (23.9) | 12 (34.3) | 87.6 | 123 (21.6) | 7 (25) | 94.6 | 301 (9) | 14 (9) | 95.6 | 156 (27.5) | 14 (33.3) | 91.8 | 37 (22.7) | 3 (37.5) | 92.5 |
| ≥1975 | 54 (15.2) | 3 (8.6) | 94.7 | 37 (6.5) | 2 (7.1) | 94.9 | 154 (4.6) | 6 (3.9) | 96.3 | 111 (19.6) | 13 (31) | 89.5 | 27 (16.6) | 1 (12.5) | 96.4 |
| Age | |||||||||||||||
| <50 years | 118 (33.2) | 13 (37.1) | 90.1 | 135 (23.7) | 7 (25) | 95.1 | 352 (10.5) | 14 (9) | 96.2 | 222 (39.2) | 25 (59.5) | 89.9 | 53 (32.5) | 4 (50) | 93 |
| 50‐60 | 162 (45.6) | 17 (48.6) | 90.5 | 277 (48.6) | 16 (57.1) | 94.5 | 1,356 (40.4) | 47 (30.3) | 96.7 | 261 (46) | 12 (28.6) | 95.6 | 82 (50.3) | 3 (37.5) | 96.5 |
| ≥60 | 75 (21.1) | 5 (14.3) | 93.8 | 158 (27.7) | 5 (17.9) | 96.9 | 1,652 (49.2) | 94 (60.6) | 94.6 | 84 (14.8) | 5 (11.9) | 94.4 | 28 (17.2) | 1 (12.5) | 96.6 |
| Sex | |||||||||||||||
| Female | 108 (30.4) | 9 (25.7) | 92.3 | 205 (36) | 8 (28.6) | 96.2 | 1,136 (33.8) | 31 (20) | 97.3 | 203 (35.8) | 16 (38.1) | 92.7 | 44 (27) | 1 (12.5) | 97.8 |
| Male | 247 (69.6) | 26 (74.3) | 90.5 | 365 (64) | 20 (71.4) | 94.8 | 2,224 (66.2) | 124 (80) | 94.7 | 364 (64.2) | 26 (61.9) | 93.3 | 119 (73) | 7 (87.5) | 94.4 |
| Ethnicity | |||||||||||||||
| White | 342 (96.3) | 34 (97.1) | 91 | 552 (96.8) | 27 (96.4) | 95.3 | 3,058 (91) | 144 (92.9) | 95.5 | 556 (98.1) | 40 (95.2) | 93.3 | 150 (92) | 8 (100) | 94.9 |
| Others | 13 (3.7) | 1 (2.9) | 92.9 | 18 (3.2) | 1 (3.6) | 94.7 | 302 (9) | 11 (7.1) | 96.5 | 11 (1.9) | 2 (4.8) | 84.6 | 13 (8) | 0 (0) | 100 |
| Treatment duration | |||||||||||||||
| <8 weeks | 1 (0.3) | 3 (8.6) | 25 | 4 (0.7) | 1 (3.6) | 80 | 7 (0.2) | 5 (3.2) | 58.3 | 5 (0.9) | 1 (2.4) | 83.3 | |||
| 8 weeks | 66 (18.6) | 8 (22.9) | 89.2 | 77 (13.5) | 7 (25) | 91.7 | 663 (19.7) | 32 (20.6) | 95.4 | 85 (15) | 8 (19) | 91.4 | 25 (15.3) | 1 (12.5) | 96.2 |
| 12 weeks | 247 (69.6) | 20 (57.1) | 92.5 | 413 (72.5) | 17 (60.7) | 96 | 2,180 (64.9) | 85 (54.8) | 96.2 | 415 (73.2) | 31 (73.8) | 93 | 120 (73.6) | 7 (87.5) | 94.5 |
| >12‐<24 weeks | 9 (2.5) | 1 (2.9) | 90 | 3 (0.5) | 1 (3.6) | 75 | 49 (1.5) | 5 (3.2) | 90.7 | 7 (1.2) | 2 (4.8) | 77.8 | 1 (0.6) | 0 (0) | 100 |
| 24 weeks | 32 (9) | 3 (8.6) | 91.4 | 73 (12.8) | 2 (7.1) | 97.3 | 461 (13.7) | 28 (18.1) | 94.3 | 55 (9.7) | 0 (0) | 100 | 17 (10.4) | 0 (0) | 100 |
| Previous treatment | |||||||||||||||
| No | 304 (85.6) | 29 (82.9) | 91.3 | 446 (78.2) | 24 (85.7) | 94.9 | 2,650 (78.9) | 112 (72.3) | 95.9 | 485 (85.5) | 39 (92.9) | 92.6 | 142 (87.1) | 8 (100) | 94.7 |
| Yes | 51 (14.4) | 6 (17.1) | 89.5 | 124 (21.8) | 4 (14.3) | 96.9 | 710 (21.1) | 43 (27.7) | 94.3 | 82 (14.5) | 3 (7.1) | 96.5 | 21 (12.9) | 0 (0) | 100 |
| HCV RNA viral load (IU/mL) | |||||||||||||||
| <124,677 (Q1) | 92 (25.9) | 6 (17.1) | 93.9 | 149 (26.1) | 11 (39.3) | 93.1 | 817 (24.3) | 33 (21.3) | 96.1 | 147 (25.9) | 17 (40.5) | 89.6 | 41 (25.2) | 2 (25) | 95.3 |
| 124,677‐670,049 (Q2) | 103 (29) | 11 (31.4) | 90.4 | 119 (20.9) | 7 (25) | 94.4 | 826 (24.6) | 40 (25.8) | 95.4 | 151 (26.6) | 9 (21.4) | 94.4 | 47 (28.8) | 1 (12.5) | 97.9 |
| 670,049‐2,212,170 (Q3) | 74 (20.8) | 7 (20) | 91.4 | 147 (25.8) | 2 (7.1) | 98.7 | 880 (26.2) | 40 (25.8) | 95.7 | 112 (19.8) | 10 (23.8) | 91.8 | 42 (25.8) | 2 (25) | 95.5 |
| ≥2,212,170 (Q4) | 86 (24.2) | 11 (31.4) | 88.7 | 152 (26.7) | 6 (21.4) | 96.2 | 819 (24.4) | 41 (26.5) | 95.2 | 157 (27.7) | 6 (14.3) | 96.3 | 33 (20.2) | 3 (37.5) | 91.7 |
| Missing | 3 (0.5) | 2 (7.1) | 60 | 18 (0.5) | 1 (0.6) | 94.7 | |||||||||
| Diabetes | |||||||||||||||
| No | 317 (89.3) | 30 (85.7) | 91.4 | 516 (90.5) | 25 (89.3) | 95.4 | 3,044 (90.6) | 135 (87.1) | 95.8 | 517 (91.2) | 40 (95.2) | 92.8 | 161 (98.8) | 8 (100) | 95.3 |
| Yes | 38 (10.7) | 5 (14.3) | 88.4 | 54 (9.5) | 3 (10.7) | 94.7 | 316 (9.4) | 20 (12.9) | 94 | 50 (8.8) | 2 (4.8) | 96.2 | 2 (1.2) | 0 (0) | 100 |
| Cirrhosis | |||||||||||||||
| No | 320 (90.1) | 31 (88.6) | 91.2 | 507 (88.9) | 26 (92.9) | 95.1 | 3,010 (89.6) | 127 (81.9) | 96 | 526 (92.8) | 41 (97.6) | 92.8 | 157 (96.3) | 8 (100) | 95.2 |
| Yes | 35 (9.9) | 4 (11.4) | 89.7 | 63 (11.1) | 2 (7.1) | 97 | 350 (10.4) | 28 (18.1) | 92.6 | 41 (7.2) | 1 (2.4) | 97.6 | 6 (3.7) | 0 (0) | 100 |
| Decompensated cirrhosis | |||||||||||||||
| No | 338 (95.2) | 33 (94.3) | 91.1 | 537 (94.2) | 27 (96.4) | 95.2 | 3,182 (94.7) | 142 (91.6) | 95.7 | 539 (95.1) | 41 (97.6) | 92.9 | 161 (98.8) | 8 (100) | 95.3 |
| Yes | 17 (4.8) | 2 (5.7) | 89.5 | 33 (5.8) | 1 (3.6) | 97.1 | 178 (5.3) | 13 (8.4) | 93.2 | 28 (4.9) | 1 (2.4) | 96.6 | 2 (1.2) | 0 (0) | 100 |
| HBV | |||||||||||||||
| No | 307 (86.5) | 31 (88.6) | 90.8 | 499 (87.5) | 27 (96.4) | 94.9 | 3,200 (95.2) | 151 (97.4) | 95.5 | 487 (85.9) | 37 (88.1) | 92.9 | 152 (93.3) | 8 (100) | 95 |
| Yes | 48 (13.5) | 4 (11.4) | 92.3 | 71 (12.5) | 1 (3.6) | 98.6 | 160 (4.8) | 4 (2.6) | 97.6 | 80 (14.1) | 5 (11.9) | 94.1 | 11 (6.7) | 0 (0) | 100 |
| HIV | |||||||||||||||
| No | 272 (76.6) | 27 (77.1) | 91 | 482 (84.6) | 25 (89.3) | 95.1 | 3,186 (94.8) | 150 (96.8) | 95.5 | 416 (73.4) | 30 (71.4) | 93.3 | 142 (87.1) | 7 (87.5) | 95.3 |
| Yes | 83 (23.4) | 8 (22.9) | 91.2 | 88 (15.4) | 3 (10.7) | 96.7 | 174 (5.2) | 5 (3.2) | 97.2 | 151 (26.6) | 12 (28.6) | 92.6 | 21 (12.9) | 1 (12.5) | 95.5 |
| Problematic alcohol use | |||||||||||||||
| Recent | 145 (40.8) | 9 (25.7) | 94.2 | 63 (11.1) | 2 (7.1) | 96.9 | 141 (4.2) | 10 (6.5) | 93.4 | 97 (17.1) | 5 (11.9) | 95.1 | 7 (4.3) | 1 (12.5) | 87.5 |
| Past | 89 (25.1) | 9 (25.7) | 90.8 | 257 (45.1) | 10 (35.7) | 96.3 | 327 (9.7) | 12 (7.7) | 96.5 | 199 (35.1) | 12 (28.6) | 94.3 | 28 (17.2) | 0 (0) | 100 |
| None | 121 (34.1) | 17 (48.6) | 87.7 | 250 (43.9) | 16 (57.1) | 94 | 2,892 (86.1) | 133 (85.8) | 95.6 | 271 (47.8) | 25 (59.5) | 91.6 | 128 (78.5) | 7 (87.5) | 94.8 |
| Major mental illness | |||||||||||||||
| No | 119 (33.5) | 13 (37.1) | 90.2 | 220 (38.6) | 12 (42.9) | 94.8 | 2,747 (81.8) | 125 (80.6) | 95.6 | 233 (41.1) | 15 (35.7) | 94 | 128 (78.5) | 8 (100) | 94.1 |
| Yes | 236 (66.5) | 22 (62.9) | 91.5 | 350 (61.4) | 16 (57.1) | 95.6 | 613 (18.2) | 30 (19.4) | 95.3 | 334 (58.9) | 27 (64.3) | 92.5 | 35 (21.5) | 0 (0) | 100 |
| Elixhauser index* | |||||||||||||||
| 0 | 41 (11.5) | 5 (14.3) | 89.1 | 47 (8.2) | 2 (7.1) | 95.9 | 1,640 (48.8) | 65 (41.9) | 96.2 | 65 (11.5) | 6 (14.3) | 91.5 | 112 (68.7) | 7 (87.5) | 94.1 |
| ≥1 | 314 (88.5) | 30 (85.7) | 91.3 | 523 (91.8) | 26 (92.9) | 95.3 | 1,720 (51.2) | 90 (58.1) | 95 | 502 (88.5) | 36 (85.7) | 93.3 | 51 (31.3) | 1 (12.5) | 98.1 |
| Material deprivation | |||||||||||||||
| Q1 (most privileged) | 64 (18) | 6 (17.1) | 91.4 | 78 (13.7) | 2 (7.1) | 97.5 | 485 (14.4) | 28 (18.1) | 94.5 | 114 (20.1) | 6 (14.3) | 95 | 25 (15.3) | 0 (0) | 100 |
| Q2 | 42 (11.8) | 3 (8.6) | 93.3 | 80 (14) | 4 (14.3) | 95.2 | 641 (19.1) | 26 (16.8) | 96.1 | 72 (12.7) | 5 (11.9) | 93.5 | 18 (11) | 3 (37.5) | 85.7 |
| Q3 | 53 (14.9) | 3 (8.6) | 94.6 | 101 (17.7) | 5 (17.9) | 95.3 | 730 (21.7) | 30 (19.4) | 96.1 | 91 (16) | 6 (14.3) | 93.8 | 31 (19) | 0 (0) | 100 |
| Q4 | 81 (22.8) | 10 (28.6) | 89 | 150 (26.3) | 9 (32.1) | 94.3 | 746 (22.2) | 33 (21.3) | 95.8 | 99 (17.5) | 9 (21.4) | 91.7 | 42 (25.8) | 1 (12.5) | 97.7 |
| Q5 (most deprived) | 114 (32.1) | 13 (37.1) | 89.8 | 156 (27.4) | 8 (28.6) | 95.1 | 725 (21.6) | 35 (22.6) | 95.4 | 177 (31.2) | 15 (35.7) | 92.2 | 44 (27) | 4 (50) | 91.7 |
| Unknown | 1 (0.3) | 0 (0) | 100 | 5 (0.9) | 0 (0) | 100 | 33 (1) | 3 (1.9) | 91.7 | 14 (2.5) | 1 (2.4) | 93.3 | 3 (1.8) | 0 (0) | 100 |
| Social deprivation | |||||||||||||||
| Q1 (most privileged) | 22 (6.2) | 3 (8.6) | 88 | 51 (8.9) | 3 (10.7) | 94.4 | 378 (11.3) | 22 (14.2) | 94.5 | 39 (6.9) | 0 (0) | 100 | 22 (13.5) | 1 (12.5) | 95.7 |
| Q2 | 34 (9.6) | 3 (8.6) | 91.9 | 68 (11.9) | 2 (7.1) | 97.1 | 455 (13.5) | 18 (11.6) | 96.2 | 55 (9.7) | 3 (7.1) | 94.8 | 12 (7.4) | 0 (0) | 100 |
| Q3 | 33 (9.3) | 1 (2.9) | 97.1 | 89 (15.6) | 3 (10.7) | 96.7 | 663 (19.7) | 35 (22.6) | 95 | 61 (10.8) | 10 (23.8) | 85.9 | 22 (13.5) | 1 (12.5) | 95.7 |
| Q4 | 67 (18.9) | 4 (11.4) | 94.4 | 127 (22.3) | 6 (21.4) | 95.5 | 814 (24.2) | 31 (20) | 96.3 | 89 (15.7) | 5 (11.9) | 94.7 | 34 (20.9) | 0 (0) | 100 |
| Q5 (most deprived) | 198 (55.8) | 24 (68.6) | 89.2 | 230 (40.4) | 14 (50) | 94.3 | 1,017 (30.3) | 46 (29.7) | 95.7 | 309 (54.5) | 23 (54.8) | 93.1 | 70 (42.9) | 6 (75) | 92.1 |
| Unknown | 1 (0.3) | 0 (0) | 100 | 5 (0.9) | 0 (0) | 100 | 33 (1) | 3 (1.9) | 91.7 | 14 (2.5) | 1 (2.4) | 93.3 | 3 (1.8) | 0 (0) | 100 |
| Treatment year | |||||||||||||||
| 2010‐2014 | 5 (1.4) | 0 (0) | 100 | 6 (1.1) | 0 (0) | 100 | 73 (2.2) | 3 (1.9) | 96.1 | 4 (0.7) | 0 (0) | 100 | |||
| 2015‐2017 | 350 (98.6) | 35 (100) | 90.9 | 564 (98.9) | 28 (100) | 95.3 | 3,287 (97.8) | 152 (98.1) | 95.6 | 563 (99.3) | 42 (100) | 93.1 | 163 (100) | 8 (100) | 95.3 |
| Treatment type | |||||||||||||||
| LDV/SOF | 210 (59.2) | 17 (48.6) | 92.5 | 364 (63.9) | 11 (39.3) | 97.1 | 2,264 (67.4) | 104 (67.1) | 95.6 | 336 (59.3) | 15 (35.7) | 95.7 | 89 (54.6) | 3 (37.5) | 96.7 |
| LDV/SOF + RBV | 3 (0.8) | 0 (0) | 100 | 6 (1.1) | 0 (0) | 100 | 50 (1.5) | 2 (1.3) | 96.2 | 4 (0.7) | 0 (0) | 100 | 4 (2.5) | 0 (0) | 100 |
| SOF/VEL | 128 (36.1) | 17 (48.6) | 88.3 | 173 (30.4) | 12 (42.9) | 93.5 | 923 (27.5) | 36 (23.2) | 96.2 | 194 (34.2) | 23 (54.8) | 89.4 | 64 (39.3) | 4 (50) | 94.1 |
| SOF/VEL + RBV | 14 (3.9) | 1 (2.9) | 93.3 | 27 (4.7) | 5 (17.9) | 84.4 | 123 (3.7) | 13 (8.4) | 90.4 | 33 (5.8) | 4 (9.5) | 89.2 | 6 (3.7) | 1 (12.5) | 85.7 |
| Genotype | |||||||||||||||
| Genotype 1 | 243 (68.5) | 24 (68.6) | 91 | 430 (75.4) | 17 (60.7) | 96.2 | 2,591 (77.1) | 119 (76.8) | 95.6 | 399 (70.4) | 28 (66.7) | 93.4 | 117 (71.8) | 5 (62.5) | 95.9 |
| Genotype 2 | 19 (5.4) | 1 (2.9) | 95 | 38 (6.7) | 1 (3.6) | 97.4 | 256 (7.6) | 9 (5.8) | 96.6 | 22 (3.9) | 1 (2.4) | 95.7 | 3 (1.8) | 1 (12.5) | 75 |
| Genotype 3 | 82 (23.1) | 9 (25.7) | 90.1 | 83 (14.6) | 9 (32.1) | 90.2 | 348 (10.4) | 23 (14.8) | 93.8 | 123 (21.7) | 12 (28.6) | 91.1 | 39 (23.9) | 2 (25) | 95.1 |
| Other/unknown | 11 (3.1) | 1 (2.9) | 91.7 | 19 (3.3) | 1 (3.6) | 95 | 165 (4.9) | 4 (2.6) | 97.6 | 23 (4.1) | 1 (2.4) | 95.8 | 4 (2.5) | 0 (0) | 100 |
Elixhauser comorbidity index measures the number of comorbidities in the administrative data set.
SVR did not significantly differ across any variable among the off‐OAT/RIDU group, although it was higher among those treated with LDV/SOF compared to SOF/VEL (93% versus 89%, respectively; P = 0.19). SVR was similar among those with and without HIV infection (91% versus 91%, respectively), problematic alcohol use (93% versus 88%), or major mental illness (92% versus 90%) (Fig. 2).
Figure 2.
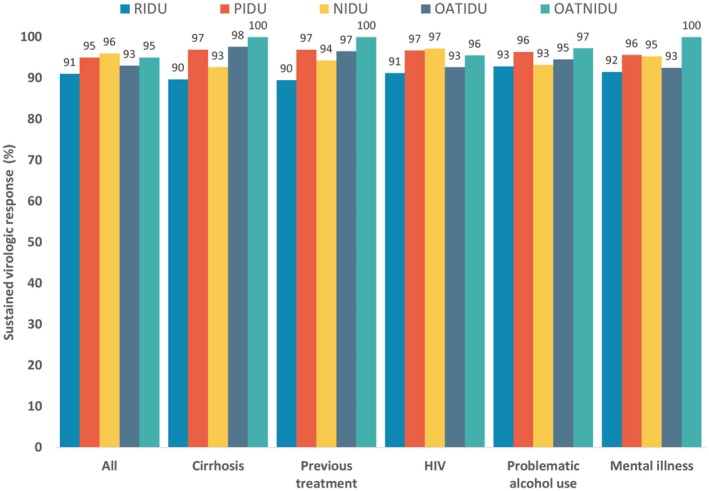
SVR by injection drug use and OAT status among patients treated with LDV/SOF and SOF/VEL.
Among the off‐OAT/PIDU group, SVR was significantly higher in those treated with LDV/SOF than SOF/VEL (97% versus 92%, respectively; P = 0.006). SVR was similar among those with and without HIV infection (97% versus 95%, respectively), problematic alcohol use (99% versus 95%), or major mental illness (96% versus 95%).
Among the off‐OAT/NIDU, SVR was higher among female patients compared to male patients (97% versus 95%; P < 0.001), birth cohort younger than 1945 compared to those born after 1945 (96% versus 91%; P < 0.02), without cirrhosis compared to those with cirrhosis (96% versus 93%; P = 0.003), and treatment‐naive patients compared to those who have been treated previously (96% versus 94%; P = 0.049).
Among the on‐OAT/IDU group, SVR was higher for those ≥50 years of age (95% versus 90% for those <50 years; P = 0.032) and those treated with LDV/SOF (96% versus 89% treated with SOF/VEL; P = 0.002).
Among the on‐OAT/NIDU group, SVR did not significantly differ across the characteristics of cirrhosis, problematic alcohol use, HIV, or HBV coinfection (Fig. 2).
Factors Associated with SVR
In the overall multivariable model, the off‐OAT/RIDU group (AOR, 1.91; 95% CI, 1.26‐2.88) and on‐OAT/IDU group (AOR, 1.50; 95% CI, 1.01‐2.22) were associated with not achieving SVR compared to the off‐OAT/NIDU group (Table 3). In this model, male sex (AOR, 1.70; 95% CI, 1.27‐2.29), cirrhosis compared to no cirrhosis (AOR, 1.50; 95% CI, 0.99‐2.28), treatment duration <8 weeks compared to 12 weeks of treatment (AOR, 4.48; 95% CI, 2.58‐7.76), and treatment duration 8 weeks compared to 12 weeks of treatment (AOR, 1.89; 95% CI, 1.29‐2.76) as well as treatment with SOF/VEL compared to LDV/SOF (AOR, 1.95; 95% CI, 1.29‐2.93) were associated with not achieving SVR after adjusting for genotype, viral load, and other important covariates. The same factors were associated with not achieving SVR in the model restricted to those off OAT and the off‐OAT/NIDU group, except for SOF/VEL, which did not significantly differ from LDV/SOF in the off‐OAT/NIDU model (Table 3).
Table 3.
Multivariable Model for Factors Associated with not Achieving SVR Among PWID and/or are on OAT
| Covariate | Overall | Off‐OAT | Off‐OAT/NIDU | Off‐OAT/IDU |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| n* | 5,259 | 4,479 | 3,496 | 977 |
| Injection drug use and OAT | ||||
| Off‐OAT/RIDU | 1.91 (1.26‐2.88) | |||
| Off‐OAT/PIDU | 1.01 (0.66‐1.57) | 1.44 (1.04‐2.01)† | ||
| Off‐OAT/NIDU | 1.00 | 1.00 | ||
| On‐OAT/IDU | 1.50 (1.01‐2.22) | |||
| On‐OAT/NIDU | 1.00 (0.47‐2.1) | |||
| Age (years) | ||||
| ≤39 | 1.00 | 1.00 | 1.00 | 1.00 |
| 40‐59 | 0.65 (0.46‐0.92) | 0.77 (0.5‐1.19) | 0.76 (0.41‐1.42) | 0.86 (0.46‐1.6) |
| ≥60 | 0.88 (0.61‐1.27) | 0.99 (0.64‐1.53) | 1.2 (0.66‐2.18) | 0.47 (0.2‐1.09) |
| Sex, male | 1.7 (1.27‐2.29) | 1.91 (1.37‐2.68) | 2.11 (1.4‐3.18) | 1.53 (0.83‐2.84) |
| Previous treatment (referenced to No) | 1.08 (0.76‐1.54) | 1.13 (0.77‐1.65) | 1.16 (0.75‐1.8) | 0.93 (0.41‐2.1) |
| Cirrhosis (referenced to No) | 1.5 (0.99‐2.28) | 1.51 (0.98‐2.33) | 1.6 (0.98‐2.6) | 1.27 (0.47‐3.42) |
| HIV (referenced to No) | 0.99 (0.64‐1.53) | 0.85 (0.48‐1.49) | 0.62 (0.24‐1.57) | 1.12 (0.53‐2.39) |
| HBV (referenced to No) | 0.65 (0.37‐1.15) | 0.59 (0.29‐1.18) | 0.55 (0.2‐1.54) | 0.66 (0.25‐1.76) |
| Material deprivation | ||||
| Q1 (most privileged) | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 0.94 (0.6‐1.47) | 0.75 (0.46‐1.22) | 0.55 (0.2‐1.54) | 1.02 (0.34‐3.02) |
| Q3 | 0.86 (0.55‐1.35) | 0.76 (0.47‐1.23) | 0.69 (0.4‐1.2) | 0.9 (0.31‐2.59) |
| Q4 | 1 (0.66‐1.51) | 0.87 (0.55‐1.36) | 0.72 (0.42‐1.23) | 1.33 (0.54‐3.29) |
| Q5 (most deprived) | 1.1 (0.74‐1.64) | 0.91 (0.59‐1.42) | 0.75 (0.44‐1.27) | 1.3 (0.54‐3.12) |
| Unknown | 1.48 (0.5‐4.33) | 1.46 (0.42‐5.04) | 0.8 (0.47‐1.35) | 6.49 (2.19‐19.27) |
| Treatment duration | ||||
| <8/24 weeks | 4.48 (2.58‐7.76) | 4.93 (2.7‐9) | 4.76 (2.26‐10.01) | 6.49 (2.19‐19.27) |
| 8 weeks | 1.89 (1.29‐2.76) | 1.87 (1.24‐2.83) | 1.53 (0.94‐2.47) | 3.87 (1.64‐9.13) |
| 12 weeks | 1.00 | 1.00 | 1.00 | 1.00 |
| 24 weeks | 1.35 (0.84‐2.19) | 1.49 (0.9‐2.45) | 1.35 (0.77‐2.34) | 2.03 (0.61‐6.7) |
| Treatment type | ||||
| LDV/SOF | 1.00 | 1.00 | 1.00 | 1.00 |
| LDV/SOF + RBV | 0.68 (0.16‐2.86) | 0.66 (0.15‐2.8) | 0.65 (0.15‐2.82) | |
| SOF/VEL | 1.95 (1.29‐2.93) | 1.59 (0.99‐2.57) | 1.04 (0.56‐1.91) | 4.15 (1.73‐9.95) |
| SOF/VEL + RBV | 2.98 (1.68‐5.26) | 2.7 (1.41‐5.19) | 1.88 (0.84‐4.17) | 5.98 (1.78‐20.16) |
| Genotype | ||||
| Genotype 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| Genotype 2 | 0.55 (0.29‐1.04) | 0.61 (0.3‐1.25) | 0.87 (0.38‐2.02) | 0.3 (0.06‐1.42) |
| Genotype 3 | 0.99 (0.65‐1.5) | 1.23 (0.75‐2.03) | 1.6 (0.82‐3.12) | 0.82 (0.38‐1.79) |
| Genotypes 4‐6 | 0.52 (0.23‐1.21) | 0.57 (0.23‐1.44) | 0.62 (0.22‐1.74) | 0.52 (0.07‐4.05) |
| HCV RNA viral load (IU/mL) | ||||
| <124,677 (Q1) | 1.00 | 1.00 | 1.00 | 1.00 |
| 124,677‐670,049 (Q2) | 1.06 (0.75‐1.52) | 1.26 (0.85‐1.88) | 1.25 (0.77‐2.03) | 1.17 (0.57‐2.39) |
| 670,049‐2,212,170 (Q3) | 1.02 (0.7‐1.48) | 1.05 (0.69‐1.61) | 1.17 (0.71‐1.91) | 0.66 (0.28‐1.58) |
| ≥2,212,170 (Q4) | 1.15 (0.8‐1.66) | 1.33 (0.89‐2.01) | 1.31 (0.8‐2.14) | 1.19 (0.57‐2.49) |
We excluded 24 individuals with missing viral load values from the analysis. Results did not differ by exclusion.
Off‐OAT analysis compared IDU with no IDU.
In the multivariable model for the off‐OAT/IDU group, including all PWID, treatment duration of 8 weeks compared to 12 weeks of treatment (AOR, 3.87; 95% CI, 1.64‐9.13) and treatment with SOF/VEL compared to LDV/SOF (AOR, 4.15; 95% CI, 1.73‐9.95) were associated with not achieving SVR (Table 3). In a sensitivity analysis restricted to genotype 1, treatment with SOF/VEL compared to LDV/SOF (AOR, 1.89; 95% CI, 1.23‐2.91) was associated with not achieving SVR. In the model restricted to LDV/SOF, treatment duration of 8 weeks compared to 12 weeks of treatment (AOR, 1.58; 95% CI, 1.05‐2.38) was associated with not achieving SVR (Supporting Table S2).
Discussion
This study assessed the real‐world effectiveness of LDV/SOF for the treatment of genotype 1 and SOF/VEL for the treatment of all genotypes by IDU and OAT status. PWID were less likely to receive treatment in the interferon era, and treatment rates are still low in the DAA era.4, 7 Currently, there are restrictions in many jurisdictions across the world on treatment of HCV in PWID. In this study, we found that treatment with LDV/SOF or SOF/VEL yielded high SVR rates of 91% among off‐OAT/RIDU, 95% among off‐OAT/PIDU, and 93% among on‐OAT/IDU, which are all similar to people with no IDU history (96%). SVR was similar among individuals with and without conditions that tend to co‐occur among PWID, such as HIV, HBV coinfection, mental illness, and problematic alcohol use. Similar to individuals not injecting drugs, PWID on or off OAT and who had cirrhosis or genotype 3 or were treated with SOF/VEL were less likely to achieve SVR. In the multivariable model, after adjusting for other characteristics, off‐OAT/RIDU and on‐OAT/IDU groups were less likely to achieve SVR. Across models, male sex, cirrhosis, shorter treatment duration, and treatment with SOF/VEL were associated with not achieving SVR. Lower SVR among PWID groups and among male individuals was related to higher loss to follow‐up. Some of this loss was related to deaths from drug overdose while on treatment or before SVR was assessed. Both of these issues highlight that highly effective treatments alone would not be sufficient to achieve a high HCV cure and improvement in overall health among PWID. In summary, these findings provide real‐world evidence for successful treatment of PWID similar to those not injecting drugs but also highlight the need for additional measures to prevent loss to follow‐up and overdose‐related deaths among PWID.
Although there are limited DAA‐era real‐world effectiveness data on the treatment of PWID compared to those not injecting drugs, our findings are consistent with other studies. Studies of individuals not injecting drugs have shown similar higher SVR rates in clinical trials and real‐world settings, where SVR rates of approximately 90% to 95% have been reported.8, 20, 26 Available data from small previous studies and the recent reviews assessing treatment outcomes among PWID have shown high SVR rates; these studies include a trial among people on OAT using elbasvir/grazoprevir, which showed an SVR of 95%, and a recent trial on current PWID using SOF/VEL, which showed an SVR of 94%.8, 9, 12, 13, 14, 15, 16, 27 However, the study population in those trials was selective, as people with HIV were not included and study participants were followed more closely; therefore, loss to follow‐up was lower. The current study and previous studies provide consistent evidence on the successful treatment of PWID with various DAA regimens, although the SVR rate in our study was slightly lower among the off‐OAT/RIDU and on‐OAT/IDU groups. The difference in rate could be accounted for by deaths and loss to follow‐up. In addition, successful treatment does not provide protection from reinfection. Data on reinfection in the DAA era are emerging. We have recently shown that recent PWID had higher reinfection rates compared to past PWID and those not injecting.28 In the current study, some individuals who had a positive RNA test following a negative test while on treatment or soon after the end of treatment followed by a positive RNA test before or at 10 weeks after the end of treatment are considered to have failed treatment. Some of these treatment failures could be reinfections; however, it is not possible to distinguish relapse from reinfection without genetic sequencing. HCV reinfections could be prevented through the integration of HCV treatment in needle syringe distribution and OAT programs.29 Thus, there is a need for engagement in harm reduction and overdose prevention services to prevent reinfection and drug‐related deaths among PWID following treatment.
A large proportion of PWID have HIV coinfection, problematic alcohol use, and/or mental illness,3, 30, 31 and this real‐world study included people who had these characteristics. Such groups were excluded from clinical trials of DAA treatment efficacy due to strict eligibility criteria8, 11; thus, our cohort represents a real‐world PWID population treated with DAAs. We found that there was no difference in SVR by any of these characteristics, which is similar to the findings from others.13 However, among PWID who did not achieve SVR, there was a substantial loss to follow‐up, some of which was related to death, including drug overdose deaths, while on treatment or before assessment of SVR. Loss to follow‐up was higher among male patients, similar to a recent report from Germany on DAA treatment among people on OAT.13 This highlights two important issues affecting the overall impact of DAAs on health outcomes: loss to follow‐up and high mortality not related to HCV. With new highly effective treatments, we could assume that most people who complete treatment and are lost to follow‐up will achieve SVR. However, loss to follow‐up will limit opportunities to prevent drug‐related deaths as well as reinfection in this population. As there are limited data on loss to follow‐up, there is a need to further estimate the magnitude across different settings and patients more likely to be lost to follow‐up. These findings also suggest that although highly effective DAAs could cure HCV, they alone would not be sufficient to improve the overall health and survival of PWID. Preventing drug‐related deaths and ill health will require comprehensive services, such as social support, mental health services, addiction treatment, and management of co‐occurring infections, to address multiple comorbidities and socioeconomic vulnerabilities.
The SVR with SOF/VEL was lower than that with LDV/SOF. Although some of this difference in the SVR was related to patients with genotype 3 who had a lower SVR than those with genotype 1 with SOF/VEL (92% versus 93%, respectively), the difference in the SVR remained even in the analysis restricted to individuals of genotype 1. Patients treated with SOF/VEL may have slightly more severe disease than those treated with LDV/SOF. Although we accounted for genotype, viral load, and various comorbidities in the multivariable model, further studies are required to evaluate the difference in the SVR between LDV/SOF and VEL/SOF while accounting for patient characteristics. There is also a need for evaluations of baseline mutations in genotype 3, which make VEL less effective in certain subtypes of genotype 3.32 Similarly, we found SVR rates were significantly different between 8 weeks and 12 weeks of treatment after adjusting for viral load and genotype in the overall analysis and the analysis restricted to LDV/SOF; others have reported no difference in SVR with 8 weeks versus 12 weeks of LDV/SOF.33, 34 This also requires further evaluation while accounting for patient characteristics.
During the study period, treatment in British Columbia and the rest of Canada was based on disease severity (restricted to people with fibrosis level ≥F2), which was lifted in March 2017. Thus, characteristics of PWID treated during this period may be different from those who have not yet been treated in terms of injecting behaviors and co‐occurring problematic alcohol use, mental illness, homelessness, and socioeconomic marginalization. Treatment adherence, and hence effectiveness and loss to follow‐up, may be different in people with more chaotic lifestyles. As we transition into treating this group, there is a need for monitoring treatment effectiveness and loss to follow‐up to optimize appropriate supporting care needed to maintain high treatment effectiveness.
The study should be interpreted with the following limitations. The assessment of IDU was based on a validated algorithm with a sensitivity of 78% and specificity of 83%, with potential for misclassification.19 Because we found a high SVR and there was little difference across groups, misclassification is not expected to have a major impact on our findings. Our definition of PWID and the population included in this study could be different from that used in other studies; therefore, findings should be compared with caution. This study is based on health care utilization data, so detailed drug use‒related information, which could have contributed to the interpretation of the findings, was not available.
In conclusion, this large real‐world assessment of DAA effectiveness among PWID and those not injecting drugs by OAT status showed a high SVR with LDV/SOF or SOF/VEL overall and across various subgroups, including those with HIV coinfection, problematic alcohol use, and mental illness. These results are similar to other real‐world cohorts not primarily focused on PWID/OAT. Our findings confirm the possibility of achieving high cure rates among PWID and open up the possibility of expanding treatment to PWID with the potential to reduce onward transmission and overall HCV burden among PWID. However, loss to follow‐up and drug‐related mortality could reduce the overall cure rate and dilute the overall impact of highly effective treatments. This highlights that treatment alone would not be sufficient to achieve improvements in the survival and health of PWID but will require additional support measures to prevent drug‐related deaths as well as reinfections. Further work is needed to understand the impact of loss to follow‐up and interventions to reduce losses along the treatment cascade.
Supporting information
Acknowledgment
We are grateful for the assistance from BCCDC, PHSA Performance Measurement and Reporting, British Columbia Ministry of Health, British Columbia Vital Statistics Agency, and British Columbia Cancer Agency and their staff involved in data access and procurement and data management.
SEE EDITORIAL ON PAGE 453
Supported by the British Columbia Centre for Disease Control and the Canadian Institutes of Health Research (NHC‐142832 and PHE‐141773), the Canadian Hepatitis C Network and Michael Smith Foundation for Health Research (postdoctoral fellowship award to M.D.), and Canadian Institutes of Health Research and Canadian Hepatitis C Network (to C.R.).
British Columbia Hepatitis Testers Cohort Team: Seyed Ali Mussavi Rizi6, Mark Gilbert1‐2, and Jason Wong1‐2.
All inferences, opinions, and conclusions drawn in this modeling projection are those of the authors and do not reflect the opinions or policies of the Data Steward(s).
Potential conflict of interest: Dr. Ramji advises, is on the speaker’s bureau for, received grants from, and consults for AbbVie, Gilead, Merck, and Intercept; he is on the speaker’s bureau for Celgene; he received grants from Janssen and Assembly and has consulted for Bristol‐Myers Squibb, Janssen, and Novartis. Mr. Cook received grants from Hologic. Dr. Krajden received grants from Roche Molecular Systems, Boehringer Ingelheim, Merck, Siemens Healthcare Diagnostics, and Hologic. Dr. Yoshida received grants from Janssen, Genfit, Springbank, Hoffman LaRoche, and Boehringer Ingelheim; he advises for, consults for, and received grants from Gilead, Merck, AbbVie, and Intercept and advises and consults for Celgene.
References
- 1. Polaris Observatory HCV Collaborators . Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol Hepatol 2017;2:161‐176. [DOI] [PubMed] [Google Scholar]
- 2. Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017;5:e1192‐e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Janjua NZ, Yu A, Kuo M, Alvarez M, Cook D, Wong J, et al. Twin epidemics of new and prevalent hepatitis C infections in Canada: BC Hepatitis Testers Cohort. BMC Infect Dis 2016;16:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janjua NZ, Kuo M, Yu A, Alvarez M, Wong S, Cook D, et al. The population level cascade of care for hepatitis C in British Columbia, Canada: the BC Hepatitis Testers Cohort (BC‐HTC). EBioMedicine 2016;12:189‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grebely J, Oser M, Taylor LE, Dore GJ. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. J Infect Dis 2013;207(Suppl. 1):S19‐S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grebely J, Hajarizadeh B, Dore GJ. Direct‐acting antiviral agents for HCV infection affecting people who inject drugs. Nat Rev Gastroenterol Hepatol 2017;14:641‐651. [DOI] [PubMed] [Google Scholar]
- 7. Janjua NZ, Islam N, Wong J, Yoshida EM, Ramji A, Samji H, et al. Shift in disparities in hepatitis C treatment from interferon to DAA era: a population‐based cohort study. J Viral Hepat 2017;24:624‐630. [DOI] [PubMed] [Google Scholar]
- 8. Grebely J, Dalgard O, Conway B, Cunningham EB, Bruggmann P, Hajarizadeh B, et al.; SIMPLIFY Study Group . Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open‐label, single‐arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol 2018;3:153‐161. [DOI] [PubMed] [Google Scholar]
- 9. Dore GJ, Altice F, Litwin AH, Dalgard O, Gane EJ, Shibolet O, et al.; C‐EDGE CO‐STAR Study Group . Elbasvir‐grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med 2016;165:625‐634. [DOI] [PubMed] [Google Scholar]
- 10. Grebely J, Mauss S, Brown A, Bronowicki JP, Puoti M, Wyles D, et al. Efficacy and safety of ledipasvir/sofosbuvir with and without rbavirin in patients with chronic HCV genotype 1 infection receiving opioid substitution therapy: analysis of phase 3 ION trials. Clin Infect Dis 2016;63:1405‐1411. [DOI] [PubMed] [Google Scholar]
- 11. Saeed S, Strumpf EC, Walmsley SL, Rollet‐Kurhajec K, Pick N, Martel‐Laferriere V, et al. How generalizable are the results from trials of direct antiviral agents to people coinfected with HIV/HCV in the real world? Clin Infect Dis 2016;62:919‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bielen R, Moreno C, Van Vlierberghe H, Bourgeois S, Mulkay JP, Vanwolleghem T, et al. Belgian experience with direct acting antivirals in people who inject drugs. Drug Alcohol Depend 2017;177:214‐220. [DOI] [PubMed] [Google Scholar]
- 13. Christensen S, Buggisch P, Mauss S, Boker KHW, Schott E, Klinker H, et al. Direct‐acting antiviral treatment of chronic HCV‐infected patients on opioid substitution therapy: still a concern in clinical practice? Addiction 2018;113:868‐882. [DOI] [PubMed] [Google Scholar]
- 14. Mason K, Dodd Z, Guyton M, Tookey P, Lettner B, Matelski J, et al. Understanding real‐world adherence in the directly acting antiviral era: a prospective evaluation of adherence among people with a history of drug use at a community‐based program in Toronto, Canada. Int J Drug Policy 2017;47:202‐208. [DOI] [PubMed] [Google Scholar]
- 15. Morris L, Smirnov A, Kvassay A, Leslie E, Kavanagh R, Alexander N, et al. Initial outcomes of integrated community‐based hepatitis C treatment for people who inject drugs: findings from the Queensland Injectors' Health Network. Int J Drug Policy 2017;47:216‐220. [DOI] [PubMed] [Google Scholar]
- 16. Norton BL, Fleming J, Bachhuber MA, Steinman M, DeLuca J, Cunningham CO, et al. High HCV cure rates for people who use drugs treated with direct acting antiviral therapy at an urban primary care clinic. Int J Drug Policy 2017;47:196‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Janjua NZ, Kuo M, Chong M, Yu A, Alvarez M, Cook D, et al. Assessing hepatitis C burden and treatment effectiveness through the British Columbia Hepatitis Testers Cohort (BC‐HTC): design and characteristics of linked and unlinked participants. PLoS One 2016;11:e0150176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. British Columbia Centre on Substance Use and British Columbia Ministry of Health . A guideline for the clinical management of opioid use disorder. http://www.bccsu.ca/wp-content/uploads/2017/06/BC-OUD-Guidelines_June2017.pdf. Published June 5, 2017. Accessed November 2018.
- 19. Janjua NZ, Islam N, Kuo M, Yu A, Wong S, Butt ZA, Gilbert M, et al.; BC Hepatitis Testers Cohort Team . Identifying injection drug use and estimating population size of people who inject drugs using healthcare administrative datasets. Int J Drug Policy 2018;55:31‐39. [DOI] [PubMed] [Google Scholar]
- 20. Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real‐world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment‐naive, genotype 1 hepatitis C‐infected patients. Hepatology 2016;64:405‐414. [DOI] [PubMed] [Google Scholar]
- 21. Pampalon R, Gamache P, Hamel D. A deprivation index for health planning in Canada. Chronic Dis Can 2009;29:178a‐191a. [PubMed] [Google Scholar]
- 22. Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El‐Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther 2008;27:274‐282. [DOI] [PubMed] [Google Scholar]
- 23. Lo Re V 3rd, Lim JK, Goetz MB, Tate J, Bathulapalli H, Klein MB, et al. Validity of diagnostic codes and liver‐related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiol Drug Saf 2011;20:689‐699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McDonald SA, Hutchinson SJ, Bird SM, Mills PR, Robertson C, Dillon JF, et al. Hospitalization of hepatitis C‐diagnosed individuals in Scotland for decompensated cirrhosis: a population‐based record‐linkage study. Eur J Gastroenterol Hepatol 2010;22:49‐57. [DOI] [PubMed] [Google Scholar]
- 25. Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol 2013;47:e50‐e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Effectiveness of sofosbuvir‐based regimens in genotype 1 and 2 hepatitis C virus infection in 4026 U.S. Veterans. Aliment Pharmacol Ther 2015;42:559‐573. [DOI] [PubMed] [Google Scholar]
- 27. Hajarizadeh B, Cunningham EB, Reid H, Law M, Dore GJ, Grebely J. Direct‐acting antiviral treatment for hepatitis C among people who use or inject drugs: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol 2018;3:754‐767. [DOI] [PubMed] [Google Scholar]
- 28. Rossi C, Butt ZA, Wong S, Buxton JA, Islam N, Yu A, et al.; BC Hepatitis Testers Cohort Team . Hepatitis C virus reinfection after successful treatment with direct‐acting antiviral therapy in a large population‐based cohort. J Hepatol 2018;69:1007‐1014. [DOI] [PubMed] [Google Scholar]
- 29. Islam N, Krajden M, Shoveller J, Gustafson P, Gilbert M, Buxton JA, Wong J, et al.; British Columbia Hepatitis Testers Cohort (BC‐HTC) team . Incidence, risk factors, and prevention of hepatitis C reinfection: a population‐based cohort study. Lancet. Gastroenterol Hepatol 2017;2:200‐210. [DOI] [PubMed] [Google Scholar]
- 30. Butt ZA, Shrestha N, Wong S, Kuo M, Gesink D, Gilbert M, et al.; BC Hepatitis Testers Cohort . A syndemic approach to assess the effect of substance use and social disparities on the evolution of HIV/HCV infections in British Columbia. PLoS One 2017;12:e0183609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McKee G, Butt ZA, Wong S, Salway T, Gilbert M, Wong J, et al. Syndemic characterization of HCV, HBV, and HIV co‐infections in a large population based cohort study. EClinicalMedicine 2018;4:99‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith D, Magri A, Bonsall D, Ip CLC, Trebes A, Brown A, et al.; STOP‐HCV Consortium . Resistance analysis of genotype 3 hepatitis C virus indicates subtypes inherently resistant to nonstructural protein 5A inhibitors. Hepatology 2018; 10.1002/hep.29837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marcus JL, Hurley LB, Chamberland S, Champsi JH, Gittleman LC, Korn DG, et al. No difference in effectiveness of 8 vs 12 weeks of ledipasvir and sofosbuvir for treatment of hepatitis C in black patients. Clin Gastroenterol Hepatol 2018;16:927‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Curry MP, Tapper EB, Bacon B, Dieterich D, Flamm SL, Guest L, et al. Effectiveness of 8‐ or 12‐weeks of ledipasvir and sofosbuvir in real‐world treatment‐naive, genotype 1 hepatitis C infected patients. Aliment Pharmacol Ther 2017;46:540‐548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


