FIGURE 11.
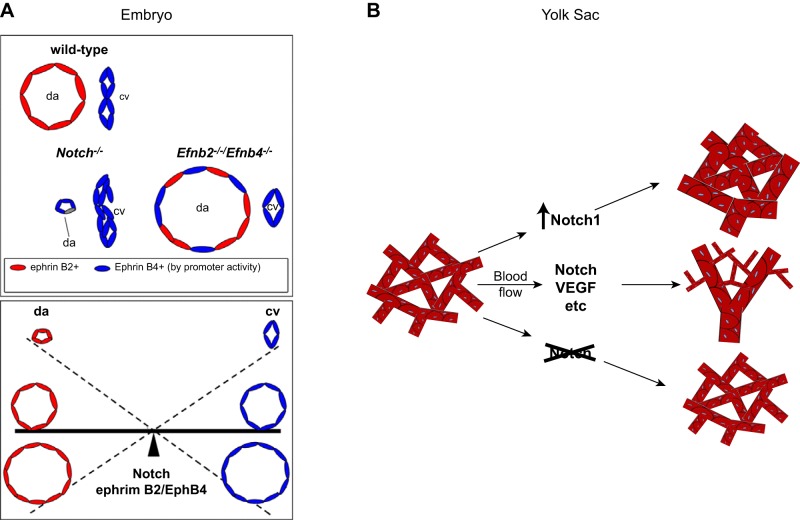
The role of Notch signaling in balancing the artery vs. vein decision during vasculogenesis in the embryo and in the yolk sac. A, top: the relative size of the dorsal aorta and cardinal vein in a wild-type embryo is schematized. In mice lacking Notch1, the endothelial arterial precursor cells (aPCs) fail to attain their arterial differentiation state, including the proper level of ephrinB2 (Efnb2) expression, which is necessary to allow sorting out of the aPCs and venous precursors (vPC). This results in a rudimentary dorsal aorta (da) with more cells attaining a vein-like fate [bottom left, cardinal vein (cv)]. In the absence of both ephrinB2 and EphB4, an enlarged dorsal aorta develops, most likely as a consequence of the improper sorting of both aPCs and vPCs into the dorsal aorta. Bottom: a seesaw model of the reciprocal relationships between appropriate arterial and venous cell sorting, which depends on Notch and ephrinB2/EphB4 signaling and controls the size of the dorsal aorta and cardinal vein. B: a similar situation is encountered in yolk sac vasculogenesis, where increased Notch signaling results in a predominance of large-caliber arterial vessels, whereas inactivation results in a predominance of small-caliber veinlike vessels. The normal wild-type situation results in development of both types of vessels that provide normal circulation in the yolk sac. [A is adapted from Kim et al. (128), with permission from Development; B is from Copeland et al. (41), with permission from BMC Dev Biol.]