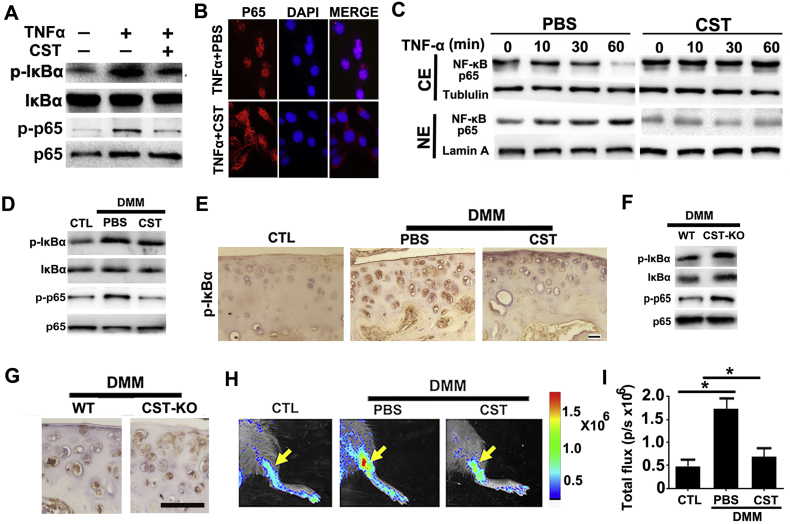
Fig. 6.
CST antagonizes NF-κB signaling pathway activation in OA. (A) CST attenuated pIκB-α and p-p65 as detected by western blotting after 12-h-stimulation of TNF-α. (B) CST antagonized nuclear translocation of NF-κB p65 in chondrocytes as determined through cell immunostaining after 1-h-stimulation of TNF-α. (C) Nuclear transaction of NF-κB p65 was analyzed by Western blotting. Western blot of NF-κB p65 was performed using cytoplasmic (CE) and nuclear (NE) extracts of 10 ng/ml TNF-α-treated (for 0, 10, 30 and 60 min, respectively) chondrocytes in the presence and absence of 50 μg/ml CST. Tubulin and Lamin A served as cytoplasmic and nuclear controls, respectively. All the mice samples of all gene types and treatment groups in DMM models were detected at the 8th week after operation, before/after sacrifice and sample collection. (D) CST suppressed the phosphorylation of IκB-α and p65 in DMM models as determined through western blotting. (E) CST suppressed pIκB-α expression according to immunohistochemistry. (F) Deficiency of CST elevated the level of pIκB-α and p-p65 in OA cartilage as determined through western blotting. (G) Immunohistochemistry results showed increased levels of pIκB-α in CST−/− mice. (H, I) CST contributed to repression of NF-κB activation in the DMM model in NF-κB luciferase reporter mice. (*p < .05, **p < .01, ***p < .005 vs control group). n = 7 for each group, Scale bar: 150 μm.