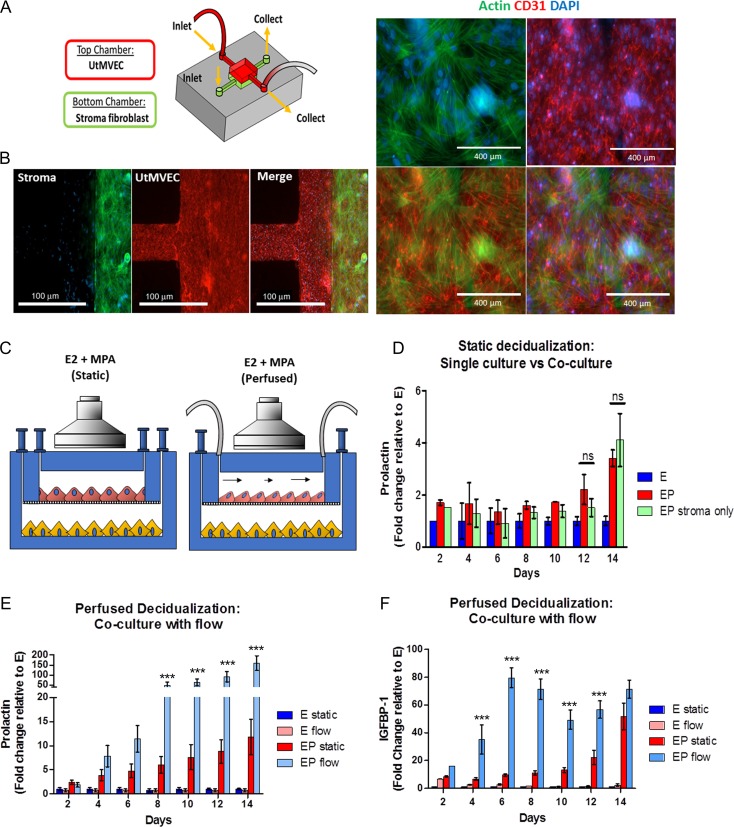
Figure 1.
Characterization of primary endometrial fibroblasts (stroma) and uterine microvascular endothelial cell (UtMVEC) co-cultures in a microfluidic model during decidualization under static and perfused conditions. (A) Schematic of the perivascular stroma model using UtMVEC and primary endometrial stromal cells with perfusion. (B) Immunofluorescence staining reveals stromal (green, actin) and UtMVEC (red, CD31) monolayers maintained in co-culture exhibit appropriate cellular morphology during long-term cultures (scale bars= 100 μm (left panel) and 400 mm (right panel)). (C) Schematic of experimental design comparing static (i.e. gravity fed) vs flow perfusion during a 14-day experiment under the influence of a synthetic progestin medroxyprogesterone acetate (MPA). (D–F) Daily samples of spent media were analyzed for decidualization markers. (D) Stromal cell prolactin (PRL) secretion was similar in cells maintained as monocultures compared to static co-culture with perivascular endothelium. (E) PRL and (F) insulin like growth factor binding protein 1 (IGFBP-1) were significantly enhanced in the perfused model compared to static co-cultures. (N = 7) E=estrogen only (E2), EP = E2 + MPA, Flow = Perfused at 1 μL/min. P values, *<0.05; ** <0.01; ***<0.001.