Fig. 1 |. treatment of GBM with PVSriPO results in extended survival.
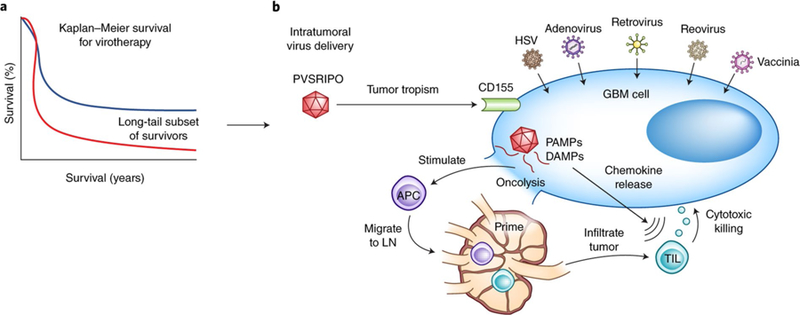
a, Many oncolytic virotherapy trials for GBM, including the one by Desjardins et al.2 using the modified polio virus PVSRIPO, demonstrate a long-tail subset of survivors beyond 2 years (blue line) not seen in historical controls (red line). b, The success of PVSRIPO as seen in the trial by Desjardins et al.2 may, in addition to direct oncolysis, be the result of viral-mediated secondary immune responses. Following intratumoral delivery of virotherapy, viral modifications permit selective GBM cell tropism (e.g., CD155 targeting by PVSRIPO). The subsequent direct viral-mediated oncolysis of tumor cells results in the release of pathogen-associated molecular patterns (PAMPs) and dying cell damage-associated molecular patterns (DAMPs) that are sensed by pattern recognition receptors on antiviral APCs, causing the APCs to mature, release proinflammatory cytokines (for example, interleukin (IL)-12, IL-1β, IL-6, and tumor necrosis factor), and migrate to draining lymph nodes (LN) for the priming of adaptive lymphocytic immune responses. Intracellular PAMP signaling is also thought to lead to upregulation of type 1 interferon pathways and chemokine release (for example, CXCL9 and CXCL10), which promote recruitment of TILs into the GBM microenvironment where they may assist with antitumoral cytotoxicity. In addition to PVSRIPO (NCT02986178*, NCT03043391*), there are many currently open virotherapy clinical trials for high grade gliomas, including several with FDA Breakthrough Therapy/Fast Track status (*) with HSV (NCT02457845, NCT03152318, NCT02062827), adenovirus (NCTs NCT02798406*, NCT03178032*, NCT03072134, NCT01811992, NCT02026271, NCT03576612), retrovirus (NCT02414165*), reovirus (NCT02444546), and vaccinia (NCT03294486) virotherapies.
