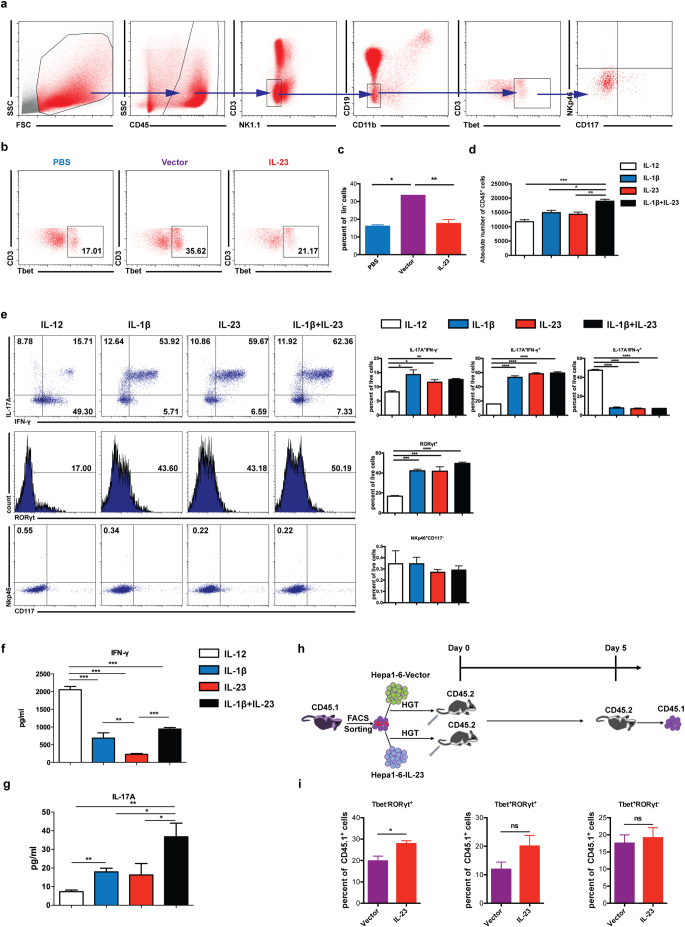
Fig. 7.
IL-23 promotes the differentiation of ILC1 to ILC3 in the tumor microenvironment. (a) 1 × 106 hepa1–6-vector cells in 2 ml PBS were injected into C57BL/6 mice hydrodynamically. 4 days later, ILC1 cells [CD45+lin(CD3,CD19,CD11b,NK1.1)−CD117−NKp46−T-bet+] were detected as shown in the flow plots. (b–c) 1 × 106 hepa1–6-IL-23, hepa1–6-vector cells in 2 ml PBS or 2 ml PBS were injected into C57BL/6 mice hydrodynamically (n = 3 mice/group). The percent of ILC1 cells in CD45+lin− population in the liver was analyzed on day 4 post injection. (d) CD127+ILC1 cells were sorted from hepa1–6 tumor-bearing mice and cultured in the presence of IL-12, IL-1β, IL-23, or IL-1β + IL-23 with IL-2 for 5 days. Cellular yield was shown at the end of the culture. (e) Flow cytometry analysis of the expression of IL-17, IFN-γ, RORγt, NKp46, and CD117 with or without various treatments was shown as dot plots or histograms (left panel) or as the percent of total live cells (right panel), respectively. (f) IFN-γ and (g) IL-17 production in the culture supernatants was measured by ELISA. (h) Experimental sketch of ILC1 adoptive transfer experiments. ILC1 cells were sorted from CD45.1+C57BL/6 tumor-bearing mice and adoptively transferred into CD45.2+C57BL/6 mice implanted with hepa1–6-IL-23 or hepa1–6-vector cells. 5 days later, the phenotypes of CD45.1+ cells in the liver were analyzed. (i) The percent of T-bet−RORγt+, T-bet+RORγt+, T-bet+RORγt− cells in CD45.1+ cells. Data are shown as mean ± SEM. One-way ANOVA followed by Tukey's multiple comparisons test (c,d,e,f,g) or Two-tail unpaired student's t-test (i) was used for statistical analysis. *p < .05, **p < .01, ***p < .001. Data shown are the representative of two independent experiments.