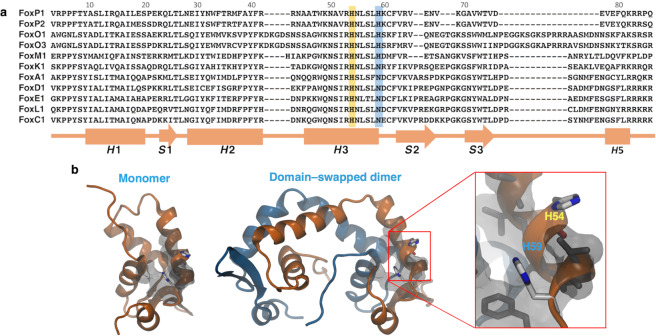
Figure 1.
Evolutionary and structural context of H59 in the DNA–binding domain of human FoxP1. (a) Sequence alignment of the DNA–binding domain from representative members of the human Fox subfamilies. Histidines highlighted in yellow and blue are the family–conserved and the M/O/P subfamily-specific residues, respectively. A secondary structure topology scheme is shown at the bottom of the sequence alignment. (b) Cartoon representation of the structure of the A39P/C61Y monomeric mutant (PDB 2KIU) and the domain–swapped dimer (generated by homology modelling using FoxP2 as template) of the forkhead domain of human FoxP1, showing the position of the two histidine residues (H54 and H59) as sticks. Hydrophobic residues surrounding H54 and H59 at 5 Å are shown in grey using their van der Waals radius.