Table 3.
Substrate Scope of Aldehydes in the Reaction with 4a
 |
 |
 |
|||
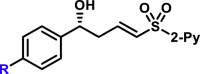
| R = H, R = F, R = Cl, R = Br, R = I, R = Me, R = tBu, R = CH3S, R = CO2Me, |
5a, 95%, 97% ee;b 5b, 88%, 97% ee; 5c, 93%, 97% ee; 5d, 85%, 92% ee; 5e, 82%, 91% ee; 5f, 96%, 97% ee; 5g, 91%, 95% ee; 5h, 90%, 97% ee; 5am, 76%, 98% ee; |
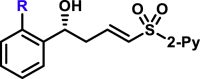
R = F, R = Br, R = Me, R = CF3, |
5K, 95%, 98% ee; 5an, 85%, 92% ee; 5ao, 98%, 99% ee; 5l, 81%, 97% ee; |
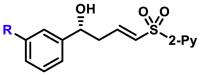
R = F, R = Cl, R = Br, |
5ap, 80%, 97% ee; 5n, 81%, 98% ee; 5o, 76%, 98% ee; |
 |
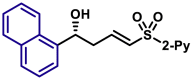 |
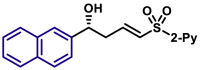
||||
| 5aq, 88%, 97% ee; | 5p, 97%, 95% ee; | ||||
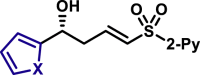 |
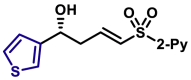 |
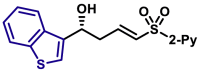 |
|||
| X = O, 5q, 81%, 98% ee; X = S, 5r, 84%, 98% ee; |
5ar, 76%, >99% ee; | 5u, 85%, 95% ee; | |||
 |
 |
 |
|||
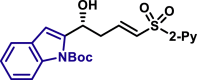
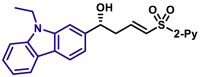
| 5as, 78%, 97% ee; | 5x, 46%, 93% ee; | R = H, 5y, 84%, 94% ee; R = Me, 5z, 51%, 95% ee; |
|||
 |
 |
||||
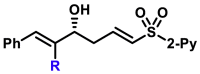
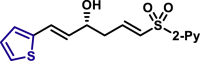
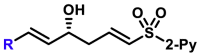
| 5aa, 80%, 92% ee; | R = Me, 5ad, 50%, 93% ee; R = nPr, 5ae, 70%, 97% ee; |
5at, 88%, 96% ee; | |||
 |
 |
||||
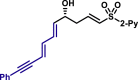
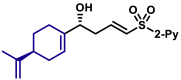
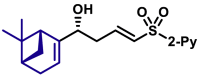
| 5au, 80%, 94% ee; | 5ah, 70%, 95% ee; | 5ak, 56%, >20/1 dr; | |||
 |
 |
 |
|||
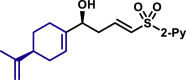
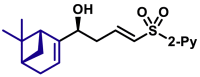
| 5ak′, 60%, >20/1 dr;c | 5al, 68%, >20/1 dr; | 5al′, 53%, 10/1 drc | |||
HPLC, high-performance liquid chromatography; NMR, nuclear magnetic resonance
2, 0.3 mmol; 4, 0.6 mmol. Isolated yield was reported. Regio- and diastereoselectivity were determined by 1H NMR analysis of reaction crude mixture. Enantioselectivity was determined by chiral-stationary-phase HPLC analysis.
Gram-scale synthesis.
(S)-DTBM-SEGPHOS was used.
