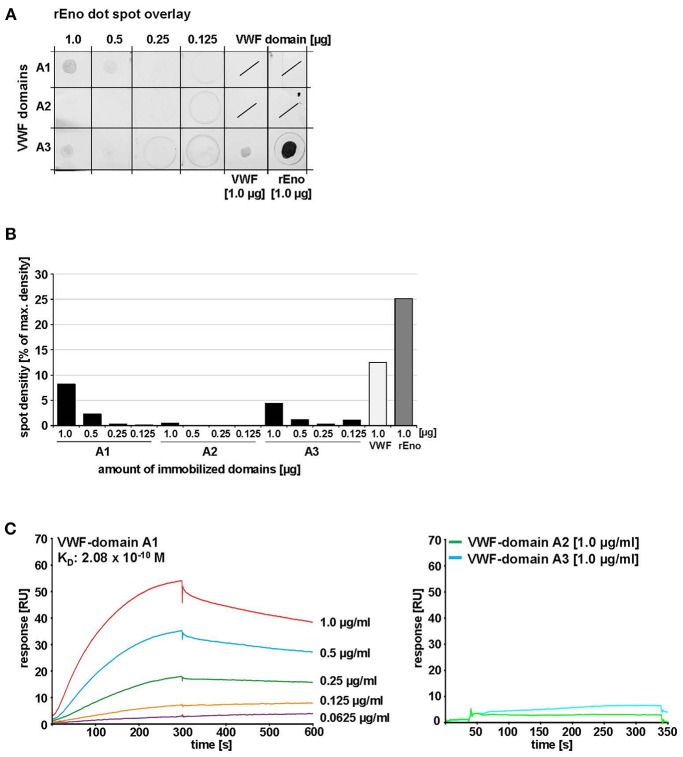
Figure 7.
Identification of VWF domains interacting with S. pneumoniae enolase. (A) Enolase protein overlay of VWF domains A1, A2, and A3 after immobilization of these domains by protein dot spotting on nitrocellulose at indicated amounts (1.0, 0.5, 0.25, and 0.125 μg). 1.0 μg VWF protein was immobilized as positive binding control and 1.0 μg rEno as antibody control. Protein overlay was performed with 1.0 μg/ml rEno and enolase-specific antibodies. Antibody overlay controls are shown in Figure S2A. (B) Densitometric analysis was performed with ImageJ version 1.49v after subtraction of antibody background. Mean density values are displayed as percent in relation to maximum density. (C) SPR analysis of immobilized rEno on Biacore CM5 sensor chips confirmed binding of VWF domain A1 to immobilized rEno, whereas no specific binding of VWF domains A2 and A3 to immobilized enolase was detected. Kinetic analyses revealed an average KD of 2.08 × 10−10 M with a Chi2-value of 2.4 for the interaction of rENo with VWF domain A1 using a Langmuir 1:1 model. Three independent binding analyses were performed with a BIACORE®T200 (GE Healthcare) at a flowrate of 10 μl/min after immobilization of 1,700 RU rEno. A representative sensorgram is shown for VWF domain A1. A coomassie stain confirming protein integrity of the A domains is added in Figure S2B.