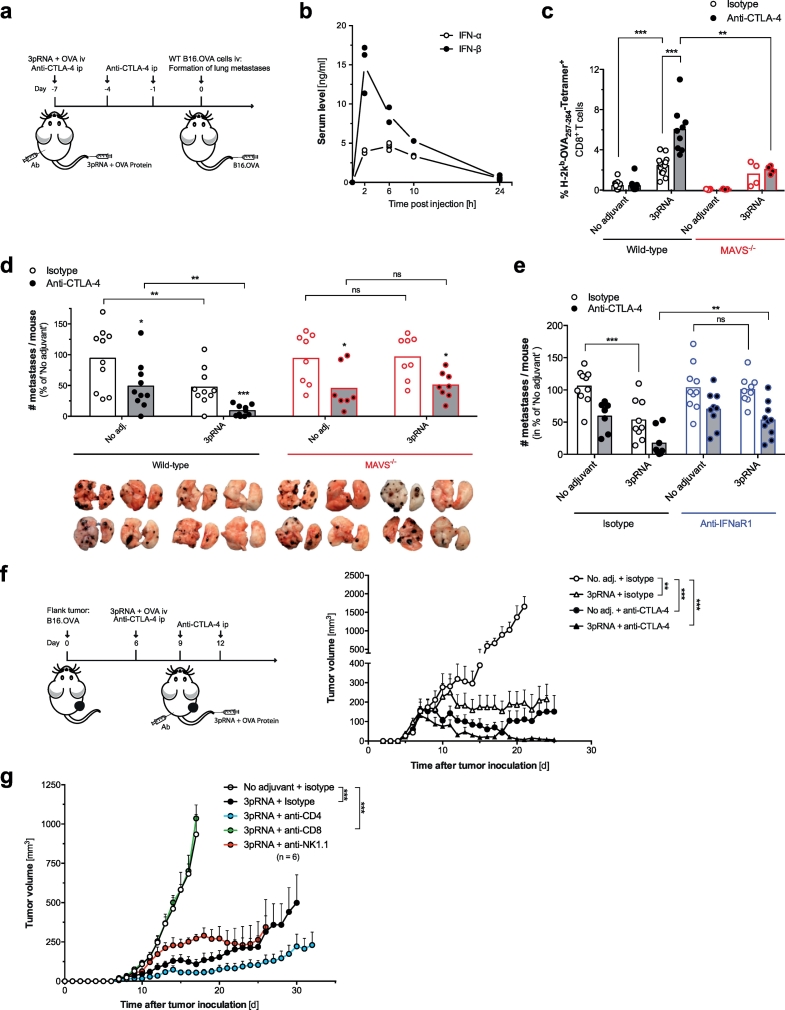
Fig. 4.
RIG-I activation synergizes with anti-CTLA-4 blockade to enhance the efficacy of anticancer vaccines. (a) ‘Prophylactic’ vaccination scheme: WT and MAVS−/− mice were vaccinated with OVA and 3pRNA iv. Anti-CTLA-4 antibody was administered ip B16.OVA tumor cells were injected iv on day 7. (b) Serum levels of IFN-I after a single 3pRNA injection. (c) Frequency of H-2Kb-SIINFEKL Tetramer+ cytotoxic T cells in peripheral blood of WT and MAVS−/− mice on day 7 after vaccination. (d) Number of macroscopically visible pseudo-metastases in the lung at day 19 after tumor induction. Data are pooled from two independent experiments and are presented as percentage of the ‘No adjuvant’ control group. (e) Number of pseudo-metastases in WT animals that were additionally injected with anti-IFNaR1 or isotype control antibody one day prior to vaccination. All data are representative of at least two independent experiments. (f) ‘Therapeutic’ vaccination scheme: WT mice were injected sc with B16.OVA cells. Intravenous vaccination with OVA and 3pRNA was performed on day 6 after tumor inoculation; anti-CTLA-4 was administered ip on day 6, 9 and 12. Tumor growth in mice was analyzed. Data show mean tumor growth ± S.E.M. from n = 5 individual mice. (g) Tumor-bearing mice were vaccinated with OVA +3pRNA as described for Fig. 4f. Some mice were additionally treated with CD4+ T-cell, CD8+ T cell or NK-cell depleting antibodies. Data give mean tumor growth ± S.E.M. of n = 6 individual mice per group. All data are representative of at least two independent experiments. (*P < 0.05, **P < 0.01, ***P < 0.001, ordinary one-way ANOVA for multiple comparisons or two-tailed unpaired t-test.)