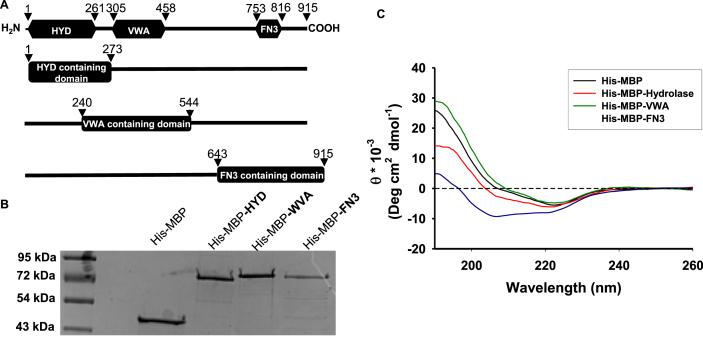
Fig. 1.
hCLCA1 domain construct design, purification, and circular dichroism spectroscopy: hCLCA1 is comprised of three functional domains (A, top): hydrolase (HYD), VWA, and FN3. Four constructs correlating to these domains were designed: amino acids 1–273 containing the hydrolase domain (His-MBP-HYD), amino acids 240–544 containing the VWA domain (His-MBP-WVA), and amino acids 643–915 containing the FN3 domain (His-MBP-FN3). His-MBP-hCLCA1 domain fusion proteins were obtained from the soluble fraction by affinity, ion exchange, and size exclusion chromatography and were assessed by SDS-PAGE (B). Structural integrity of purified His-MBP and His-MBP-hCLCA1 fusion proteins was assessed by circular dichroism spectroscopy in the far UV region from 260 to 190 nm (C).