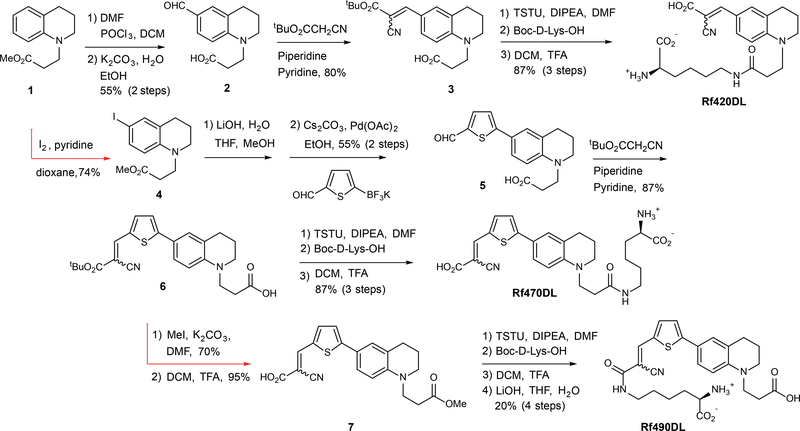
Figure 1. Synthetic routes for preparation of the RfDAAs.
Each synthetic route utilized an alkylated tetrahydroquinoline core structure (1) that incorporated an ester group to provide a functional handle for subsequent D-amino acid coupling. After Vilsmeier-Haack formylation, condensation with t-butyl cyanoacetate provided the fluorogenic fluorophore, Rf420 (3), which was coupled with D-Lys to generate Rf420DL. A similar strategy was used to prepare Rf470DL. A thiophene spacer, installed via iodination of the aryl ring and a subsequent Suzuki-Miyaura coupling reaction, was added to the tetrahydroquinoline core in order to further extend conjugation (e.g., 6). Rf490DL, a constitutional isomer of Rf470DL, was prepared by coupling D-Lys to the deprotected α-cyanoacrylic acid subunit (7).