Table 1.
Investigation on the effects of substituents on the diastereoselectivity.a
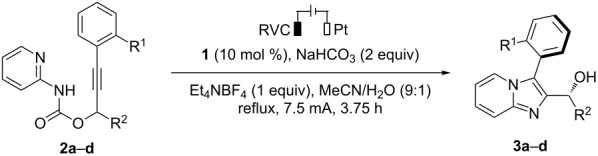 | ||
| Entry | Substrate | Product, yield,b drc |
| 1 | 2a (R1 = t-Bu, R2 = t-Bu) | (±)-3a, 68%, 14:1 dr |
| 2 | 2b (R1 = t-Bu, R2 = iPr) | (±)-3b, 64%, 3:1 dr |
| 3 | 2c (R1 = Ph, R2 = t-Bu) | (±)-3c, 73%, 2:1 dr |
| 4 | 2d (R1 = OiPr, R2 = t-Bu) | (±)-3d, 78%, 3:1 dr |
aReaction conditions: undivided cell, 1 (0.03 mmol), 2 (0.3 mmol), H2O (1 mL), MeCN (9 mL), 3.5 F mol−1. bIsolated yield. cDetermined by 1H NMR analysis of the crude reaction mixture.
