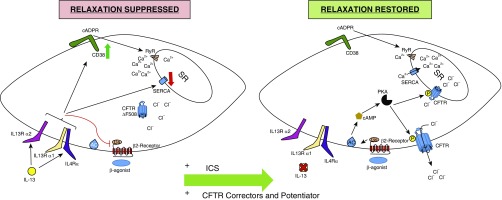
Figure 1.
Mechanisms contributing to altered airway smooth muscle contraction and relaxation in cystic fibrosis (CF). Left panel: chronic airway inflammation in CF includes IL-13, which mediates effects via dimeric receptors (IL13Rα1 with the IL4Rα or IL13R2A subunit). IL-13 induces expression of CD38, which catalyzes cyclic adenine diphosphonucleotide ribose (cADPR), which opens ryanodine receptors (RyR) to release Ca2+ from the sarcoplasmic reticulum (SR). IL-13 also reduces sarcoplasmic endoplasmic reticulum calcium ATPase (SERCA), which facilitates Ca2+ reuptake. Reduced and improperly processed CF transmembrane conductance regulator (CFTR) further limits the driving force for Ca2+ reuptake. Collectively, this leads to prolonged elevation of myoplasmic Ca2+, and increased maximum force production in response to contractile agonist stimulation. IL-13 inhibits β2-receptor-Gs–mediated adenylate cyclase activation and cAMP synthesis, which markedly reduces muscle relaxation. Right panel: effective antiinflammatory therapy (shown here as inhaled corticosteroids [ICS]) can eliminate disease-specific effects of IL-13 that inhibit bronchodilator signaling, and renew cAMP synthesis and protein kinase A (PKA) mechanisms for smooth muscle relaxation, including phosphorylation and activation of CFTR. Additionally, well-controlled inflammation prevents IL-13 effects on CD38-RyR and SERCA, resulting in reduced cytoplasmic Ca2+ and mitigation of smooth muscle hypercontractility. New triple CFTR therapy could also restore CFTR function, better enabling Ca2+ reuptake to the SR and reducing Ca2+ influx via store- and voltage-operated channels in the plasma membrane. AC = adenylyl cyclase.