Table 1.
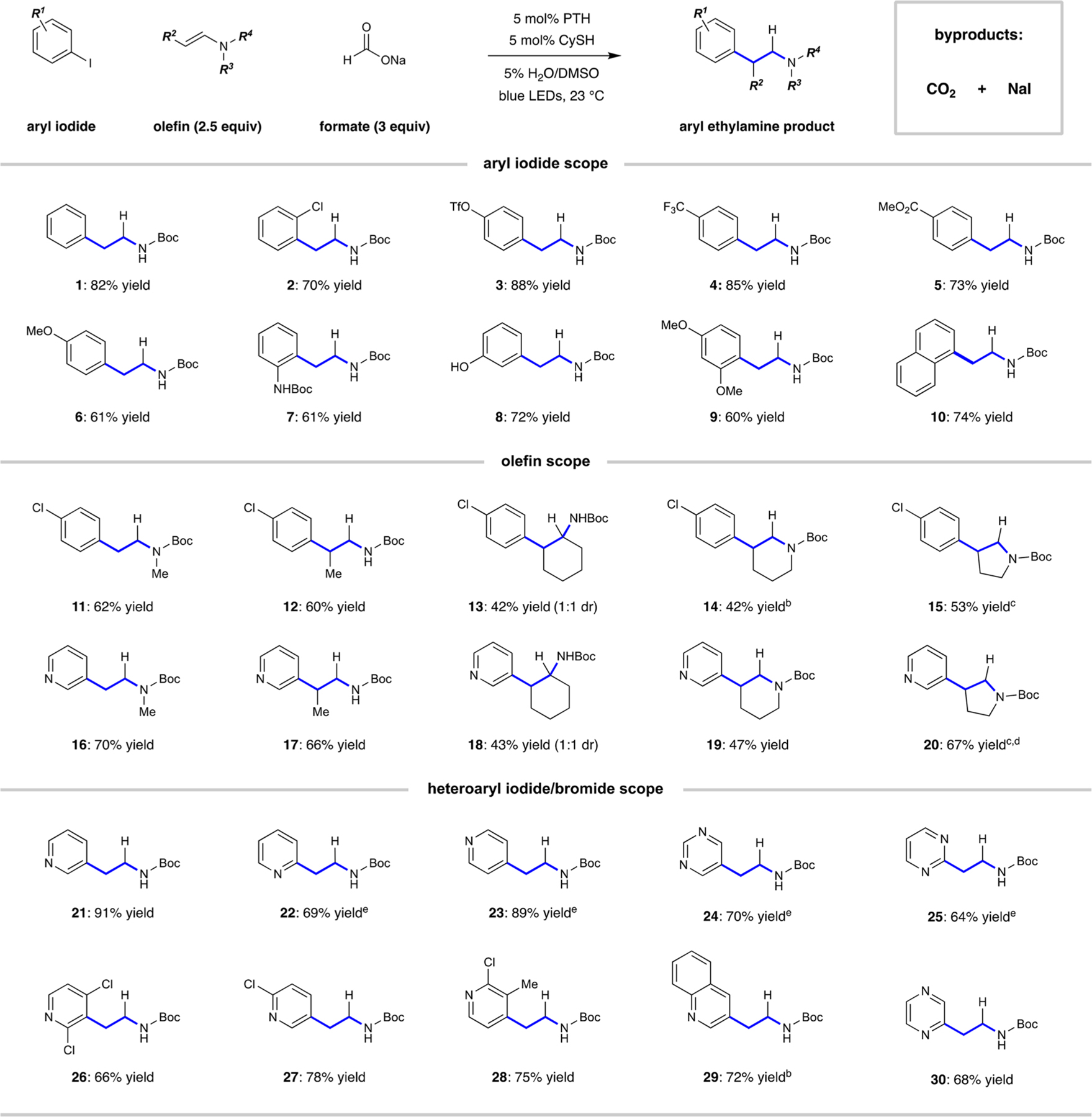 |
Reaction conditions: Iodoarene (1.0 mmol), olefin (2.5 mmol), PTH (5 mol %), CySH (5 mol %), sodium formate (3.0 mmol), 5% (v/v) H2O/ DMSO (10.0 mL), blue light, 16 h; isolated yields given.
The reactions to produce 14 and 29 were conducted on a 0.5 mmol scale (1-chloro-4-iodobenzene).
In the reactions to produce 15 and 20, N-1-naphthylphenothiazine (5 mol %) was used in place of PTH.
In the reaction to produce 20, olefin (1.0 mmol) was used as a limiting reagent with 5.0 mmol of 3-iodopyridine.
In the reactions to produce 22−25, corresponding heteroaryl bromide was used as the aryl substrate.
