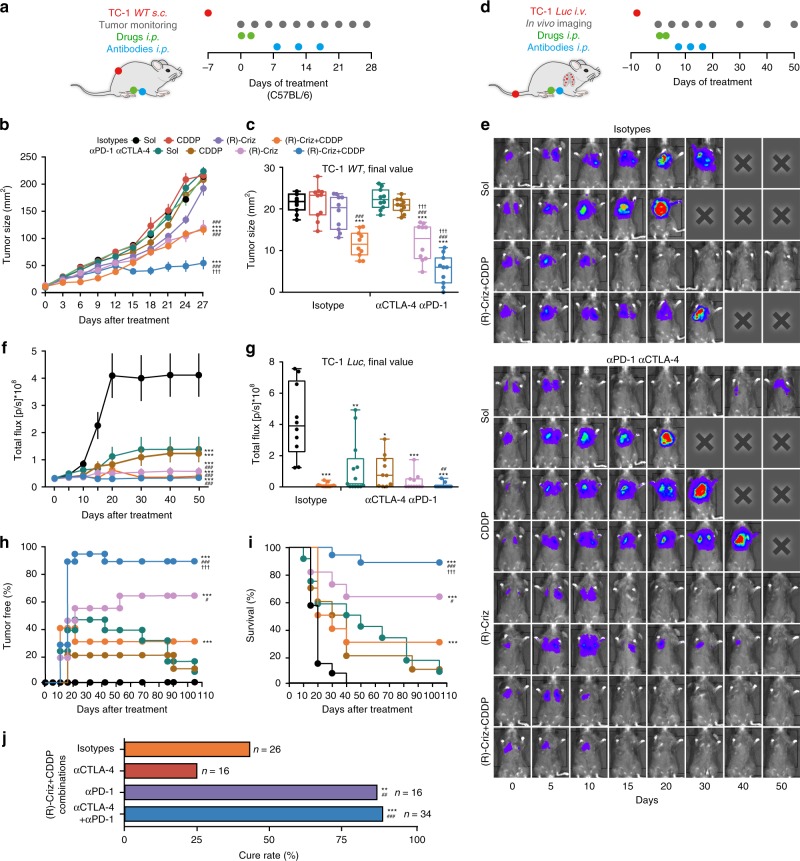
Fig. 7.
Immunogenic chemotherapy of (R)-crizotinib sensitizes NSCLC tumors to checkpoint blockades. a–c Treatment of subcutaneous (s.c.) TC1 tumors with injections of solvent control, CDDP alone or in combination with (R)-crizotinib. Isotype monocloncal antibodies (mAbs) or anti-PD-1 mAbs (αpd-1) combined with anti-CTLA-4 mAbs (αCTLA-4) were injected on day 8, 12, and 16 (schedule in a). Tumor growth was monitored (b, c) and expressed as surface (mean ± s.e.m., b) or size at endpoint (box plot, c). d–j Treatment of orthotopic TC1 Luc tumors. Once the presence of lung cancer could be detected by bioluminescence (day 0), the animals were treated according to the scheme (d). Representative images of tumor development are shown in e; average (mean ± s.e.m.) bioluminescence signals are reported in f; final values at endpoint are reported as box plot in g. The percentage of tumor free mice is reported in h; overall survival is reported in i. Data in e–i include dual checkpoint blockade (αCTLA-4/αPD-1). The effects of dual as compared to single checkpoint blockade at day 70 are shown in j. Statistical significance was calculated by means of the ANOVA Type 2 (Wald test) (b, f), ANOVA test for multiple comparisons (c, g), Likelihood ratio test (h, i) or χ2 test (j). *p < 0.05, **p < 0.01, ***p < 0.001 as compared to control group with isotype; #p < 0.05, ##p < 0.01, ###p < 0.001 as compared to control group with the combination of αPD-1 and αCTLA-4; †††p < 0.001 comparing isotype and αCTLA-4 and αPD-1 combination, n = minimum of 10 animals per group