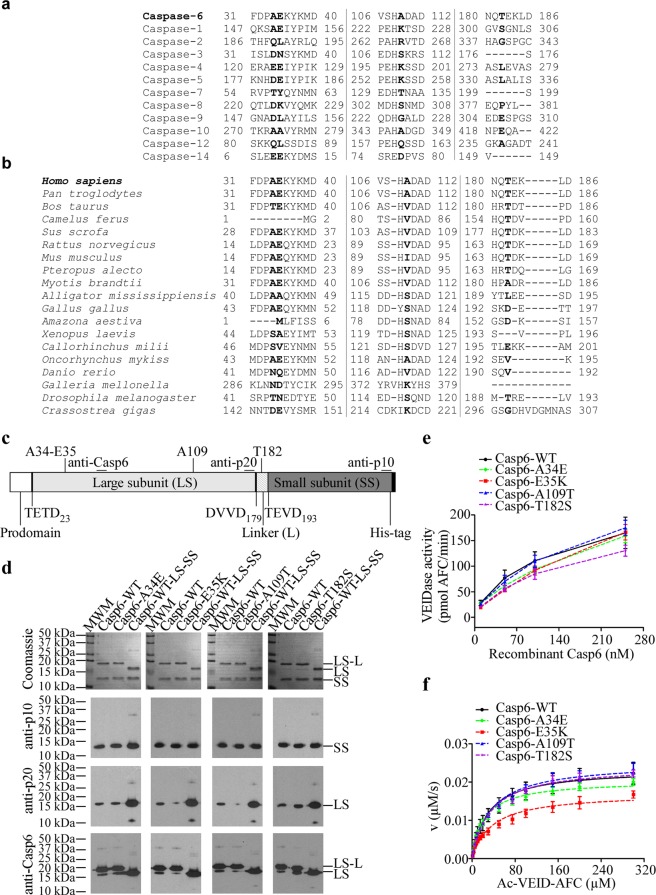
Figure 1.
Activity of rare natural Casp6 variants. (a) Protein sequence alignment of 12 human caspases (a) and Casp6 from different species (b) showing A34, E35, A109, and T182 of human Casp6 and respective homologous residues of other caspases in bold. (c) A schematic representation of full-length recombinant pro-Casp6 and its domains, the position of Casp6 processing sites (TETD23, DVVD179, TEVD193), and the epitopes recognised by antibodies. Anti-p20 is a neoepitope antibody against cleaved Casp6 at D179 position. (d) Coomassie stain of SDS-PAGE (top panel) and western-blot analyses (three bottom panels) with Casp6 Pharmingen anti-p10, neoepitope 10630 anti-p20, and Santa Cruz anti-Casp6 (LS, LS-L) antibodies or antiserum of purified recombinant caspases showing large subunit with (LS-L) and without linker (LS), and small subunit (SS) of Casp6. Cropped gels and blots are shown here for clarity, full-length gels and blots are provided in Supplementary Information Fig. S12. (e) The activity of 10–250 nM active site titrated recombinant Casp6. (f) The relationship between initial reaction velocity (v) and substrate concentration of Casp6 catalysed Ac-VEID-AFC cleavage. Data were fitted to Michaelis-Menten equation using nonlinear regression and represent the mean ± standard deviation (SD) from at least three independent experiments.