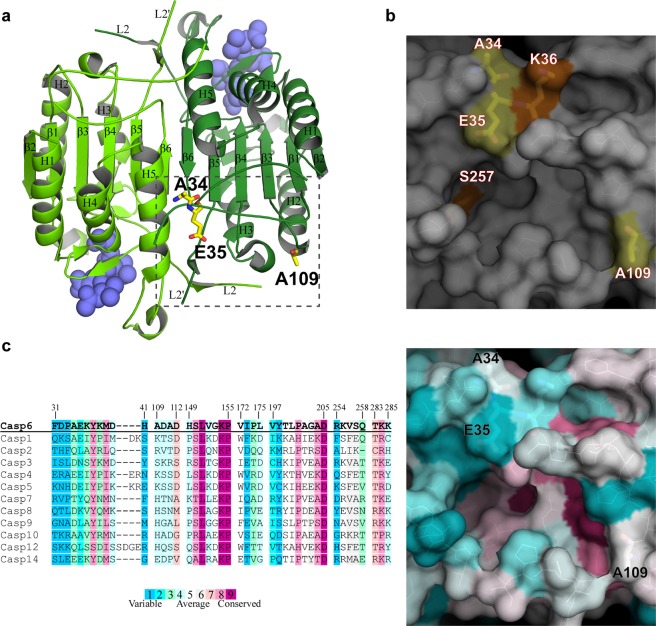
Figure 3.
Putative Casp6 allosteric pocket. (a) SNP residues A34, E35, and A109 (yellow sticks) are highlighted in the putative allosteric pocket (boxed) from one protomer of the dimeric structure of the Ac-VEID-AFC-bound Casp6-WT (PDB: 3OD5). The protomers of dimeric Casp6 are shown in green and pale green, respectively, and the active-site bound Ac-VEID-AFC molecules are represented by blue spheres. Beta strands (β1–β6), alpha helices (H1–H5), and loops L2 and L2′ are labeled in both protomers. (b) Surface representation of the putative Casp6 allosteric pocket indicating positions of A34, E35, and A109 (yellow), and Casp6 allosteric regulation sites K36 and S257 (orange). (c) Amino acid sequence conservation of the putative allosteric pocket in the human caspase family (left panel) mapped on the 3OD5 crystal structure of Casp6 (right panel).