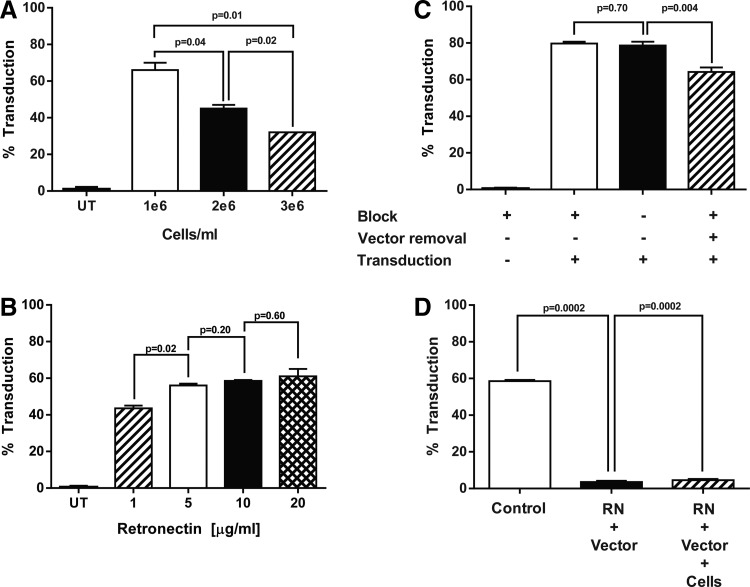
Figure 4.
Optimization of closed transduction process for the manufacture of CD19 CAR-T cells. Lymphocytes were isolated from subject apheresis products using the Sepax II and stimulated as previously described with the following changes. (A) After stimulation, PBMCs were seeded at the indicated cell density and transduced in bags as previously described. The transduction efficiency was measured by FACS staining for CD19 CAR expression 4 days post transduction. (B) Origen cell culture bags were coated with retronectin at the indicated concentrations. The next day, retronectin was removed and the bags were blocked with 2.5% human serum albumin (HSA) before vector loading. OKT3 activated-PBMCs from three patients (1 × 106 cells/mL) were added to separate bags, and transduction efficiency was measured by FACS. (C) To evaluate the need for a blocking step following retronectin coating of the bag, bags were coated with 10 μg/mL of retronectin and blocked with either 2.5% HSA or HBSS and then loaded with retroviral vector. No significant difference in transduction was observed by the addition of a blocking step. However, removal of the retroviral vector prior to the T cell transduction did significantly reduce the level of CD19 CAR expression (p = 0.004, stippled bar). (D) In the closed transduction process (control), the bag is coated with retronectin overnight followed by a wash-and-block step prior to vector loading. In an attempt to simplify the process, the block step prior to transduction was removed and the addition of retronectin and vector prior to the addition of cells was combined. In a separate effort, the addition of retronectin, vector, and cells was combined into a single step. All other conditions were the same. All data are representative of at least three independent experiments. Data are presented as mean ± SEM.