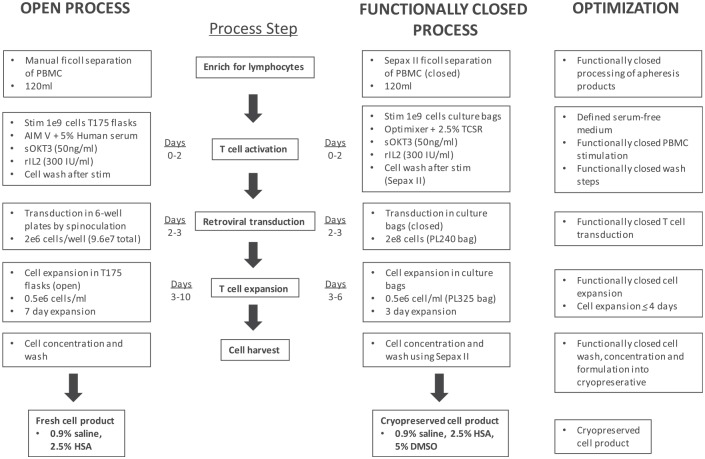
Figure 5.
Manufacturing scheme for the functionally closed production of CD19 CAR-T cells. An overview of the 6-day cell production process developed at the Surgery Branch of the National Cancer Institute. The schematic shows a comparison between the 10-day open spinoculation process for the administration of a non-cryopreserved cell product and the new 6-day closed-cell production process followed by cryopreservation of the final cell product. Optimizations achieved at each process step are indicated.