Abstract
Traumatic brain injury (TBI) frequently results in diffuse axonal injury and other white matter damage. The corpus callosum (CC) is particularly vulnerable to injury following TBI. Damage to this white matter tract has been associated with impaired neurocognitive functioning in children with TBI. Event-related potentials can identify stimulus-locked neural activity with high temporal resolution. They were used in this study to measure interhemispheric transfer time (IHTT) as an indicator of CC integrity in 44 children with moderate/severe TBI at 3–5 months post-injury, compared with 39 healthy control children. Neurocognitive performance also was examined in these groups. Nearly half of the children with TBI had IHTTs that were outside the range of the healthy control group children. This subgroup of TBI children with slow IHTT also had significantly poorer neurocognitive functioning than healthy controls—even after correction for premorbid intellectual functioning. We discuss alternative models for the relationship between IHTT and neurocognitive functioning following TBI. Slow IHTT may be a biomarker that identifies children at risk for poor cognitive functioning following moderate/severe TBI.
Key words: : corpus callosum, event-related potential, interhemispheric transfer time, neurocognitive functioning, pediatric traumatic brain injury
Introduction
Traumatic brain injury (TBI) frequently results in diffuse axonal injury, diffuse white matter atrophy, whole brain volume reductions,1 and injury to unmyelinated brain fibers.2 The corpus callosum (CC) is particularly vulnerable to diffuse axonal injury.3–7 There are conflicting results on the relation between acute injury severity and the persistence of CC injury. Reductions in CC area persisted from 3 months up through 3 years following severe pediatric TBI, while children with mild and moderate TBI had increases in CC size consistent with normal development over this time span.8 However, in a different study of children with complicated mild, moderate/severe pediatric TBI, volumetric reductions in CC were negligible at 3 months post-TBI but significant reductions were found 18 months post-injury in children with TBI.7 The reduction over time in CC volume, along with thinning of the CC, are thought to result from Wallerian degeneration following TBI.9
The posterior region, or splenium, of the CC is especially vulnerable to TBI10–13 Atrophy of the splenium of the CC has been observed following TBI.14–16 Lesions in the posterior half of the CC accounted for 80% of all CC injuries in a study of 92 patients (ages 2–77; mean age 28) with severe TBI.15 These lesions are thought to be a direct result of the injury (including diffuse axonal injury) of secondary injuries, or of impaired or arrested CC development post-injury.15 The anterior CC also is vulnerable to secondary injury mechanisms following TBI, such as elevated intracranial pressure,17 although this finding is less established in the literature. In children with severe TBI, increased intracranial pressure immediately following TBI was correlated with reduced anterior CC size and overall white matter loss 5 years post-TBI.18
Taken collectively, brain imaging studies have clearly demonstrated the presence of structural damage to the CC following TBI. The present study will test hypotheses about the functional consequences of CC damage.
Interhemispheric transfer time (IHTT) has been used to assess the functional integrity of the CC. IHTT refers to the time required for information to pass across the CC from one hemisphere to another. Patients with focal CC damage (patients with commissurotomies, callosotomies, and callosal agenesis) have slow IHTT.19,20 Disconnection in the posterior CC is associated with lower crossed–uncrossed differences in visuomotor reaction time, a measure of IHTT.21 In a case study of a patient with interhemispheric disconnection following TBI, Peru and colleagues21 suggested that the posterior CC might be a communication channel for mediating visuo-motor performance speed. The hypothesis that the posterior CC is responsible for the interhemispheric transfer of visual information is supported by other studies, including a study examining commissurotomies in non-epileptic patients22 and case studies of two patients with posterior CC sectioning for tumor removal,23 as well as in a case study of one patient with hemialexia following posterior CC surgical sectioning.24
Some evidence for impaired IHTT in TBI is provided by the performance of children with severe TBIs on a verbal dichotic listening task10 and adults on visual and tactile reaction time tasks25 that required IHTT. Although white matter atrophy was moderately related to visual and tactile reaction time task performance in adults, total CC area was not significantly related to performance on these tasks.25
The current study used electroencephalography (EEG) scalp recordings of visual event-related potentials (ERPs) to measure IHTT. This electrophysiological measure may be a more direct index of IHTT than performance on crossed-uncrossed differences (i.e., motor speed reaction time tasks) and tachistoscopic measures used in prior studies of individuals with TBI. Visual ERPs have been used in previous studies to examine IHTT as an index of CC functioning in patients with focal CC damage and in patients with CC agenesis.19,20,28 Longer IHTTs indicate slower transfer of visual information across the posterior visual brain regions.19 While previous studies have examined EEG-ERP measured IHTTs in healthy adults and adults with non-TBI CC damage, this is the first study to examine EEG-ERP measured IHTTs in a pediatric TBI sample.
There are cognitive effects of CC damage. CC atrophy following TBI is associated with impaired performance on the Wechsler Adult Intelligence Scale (WAIS) Processing Speed Index,18,25,26 the WAIS Verbal, Performance, and Full Scale IQs, Judgment of Line Orientation test, and the Trail Making Test.27 CC damage associated with elevated intracranial pressure was correlated with impairments in children's working memory and social interaction skills, and with impaired performance on the copy condition of the Rey-Osterrieth Complex Figure test.18
The current study utilized visual ERPs to measure post-acute (i.e., 3–5 months after injury) IHTTs in children with moderate-to-severe TBI (msTBI) and a group of healthy control children. In a prior paper,3 we examined the relation between visual ERP measured IHTTs and brain metabolites measured by magnetic resonance spectroscopy in 10 children with TBI post-acutely, but never compared visual ERP measured IHTTs of children with TBI to controls. Data in this study were collected from all participants on tasks tapping the neurocognitive domains, which a recent meta-analysis of studies of cognitive functioning following pediatric TBI29 found that children with msTBI had their greatest impairments. Those domains were processing speed, working memory, learning/memory, and executive functioning.
It was hypothesized that children with msTBI would have slower visual ERP IHTTs than controls. In addition, it was predicted that slow IHTTs would be associated with deficits in neurocognitive functioning, including processing speed, working memory, learning, and executive functioning in children with msTBIs.
Methods
Participants
Moderate-to-severe TBI participants were recruited from the pediatric intensive care units of four Los Angeles County trauma hospitals. The overall study was approved by the University of California, Los Angeles (UCLA) institutional review boards and the institutional review boards of each facility from which patients were recruited. Participants were invited to participate following a telephone screening for the following inclusion criteria: 1) the child had sustained a moderate-to-severe closed-head, non-penetrating TBI. Moderate-to-severe injury was defined as a Glasgow Coma Scale (GCS) score between 3 and 12 on admission to the hospital (children with higher GCS scores were included if their injury produced confirmed abnormalities on clinical brain imaging, such as hemorrhage); 2) 8–18 years of age at the time of injury; 3) normal visual acuity or normal vision once corrected with eyeglasses or contact lenses; and 4) the child's proficiency in the English language so that he/she could understand instructions and participate in the neurocognitive assessments. Participants with a pre-TBI history of developmental, neurological, or psychiatric disorders (e.g., including attention-deficit hyperactivity disorder [ADHD[ and previous head injuries) were excluded. Parents provided written informed consent, while children provided written assent to participate in this study. Magnetic resonance imaging (MRI) scans, standard neurocognitive evaluations, and another neurobehavioral measure were collected from participants in addition to the EEG/ERP data. The control group included 39 typically-developing children ascertained from the same communities as the msTBI patients. Healthy control group children had no history of head trauma and also were required to meet inclusion criteria 2–4. Participants completed the entire protocol, including electrophysiological and neurocognitive evaluations, in one day.
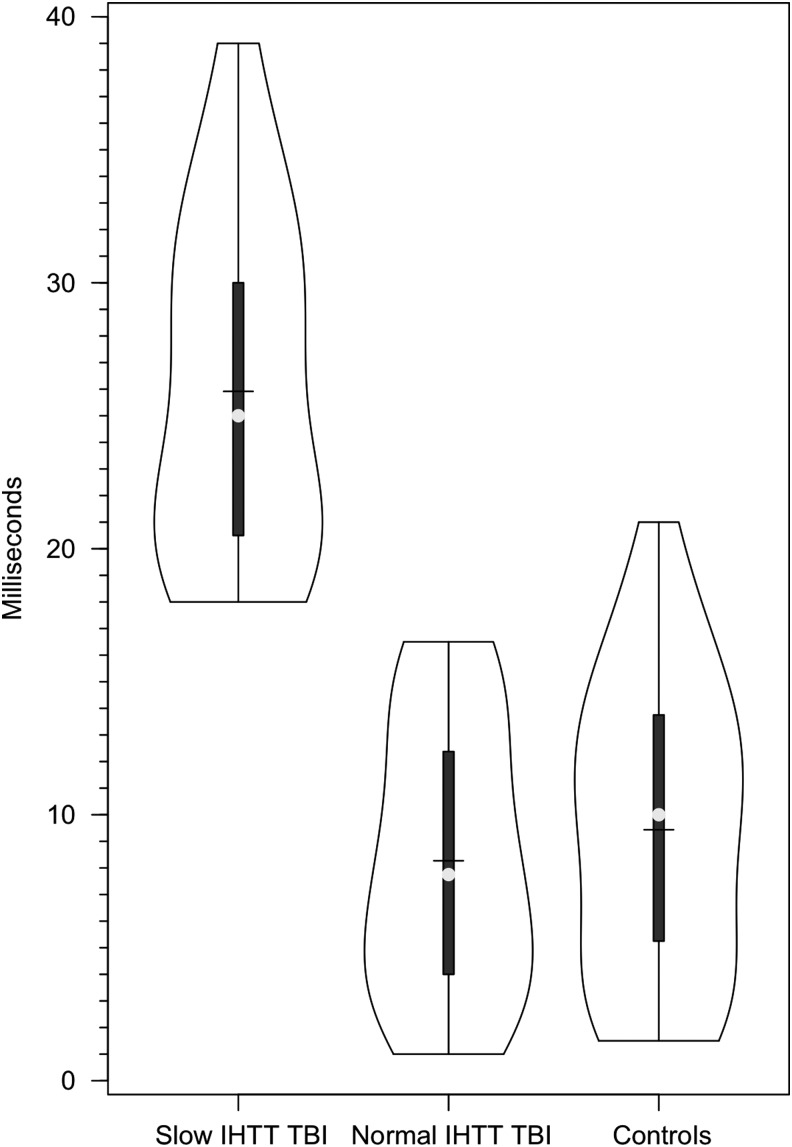
Eighty-three children were entered into this study. Six children (five children with msTBI and one control child) were removed from data analysis due to unreadable ERP results. These children's ERPs were unreadable due either to excessive eye movements, muscle tension–related artifacts, or other “noise” that made the peak points on their parietal and occipital EEG waveforms unidentifiable. Three additional children (two controls and one child with TBI) were removed from data analysis due to missing mastoid EEG channels. To remain consistent with inclusion criteria, one additional child with msTBI was removed from data analysis after we learned that this child was diagnosed with ADHD prior to the msTBI. There were 44 children with TBI and 39 healthy control participants included in the analyses presented below. Preliminary review of the distribution of IHTT scores of the msTBI and control participants (Fig. 1) revealed that the distribution of scores in the msTBI group was highly skewed, with a substantial number of the scores of the msTBI group outside of the normal range. Just over half (n = 26) of the msTBI group had IHTT scores within 1.5 standard deviations of the normal range. The balance of the TBI group had very slow IHTTs, outside of the normal range. The normal range was defined based on the IHTT scores for the healthy control group. The cutoff for including participants in the slow IHTT group was an IHTT greater than the 1.5 standard deviations of the range in the healthy controls. Consequently, the msTBI group was split into two subgroups: normal IHTT TBI (n = 26) and slow IHTT (n = 18) TBI children (see results section for more details).
FIG. 1.
Violin plots of the distribution of interhemsipheric transfer times (IHTT) in the healthy control, slow, and normal interhemispheric transfer time moderate-to-severe traumatic brain injury groups.
The demographic and clinical characteristics of the three groups are presented in Table 1. A 3 × 1 analysis of variance revealed that the normal range IHTT and slow IHTT msTBI groups and healthy control group did not significantly differ in age. Chi-square tests revealed that the three groups also did not significantly differ in gender or handedness. Hand dominance was based upon self-report from participants. The normal IHTT and slow IHTT msTBI groups did not differ in their worst GCS score recorded during the first 24 h of injury, nor were these two groups different on the average time since injury at time of study participation. Worst GCS score was not available for one normal IHTT msTBI group participant. Table 1 presents the demographic characteristics and injury severity of the groups in this study.
Table 1.
Demographics and Injury Severity by Group: Controls Versus Two TBI Groups
| Control Mean (SD) | Normal IHTT TBI Mean (SD) | Slow IHTT TBI Mean (SD) | Significance value | |
|---|---|---|---|---|
| N | 39 | 26 | 18 | |
| Age at testing (years) | 15 (3) | 14.6 (3.3) | 14.3 (2.4) | F = 0.41 (p = 0.66) |
| Gender (male) | 25 (64%) | 21 (81%) | 12 (67%) | χ2 = 2.17 (p = 0.34) |
| Handedness (right dominant) | 35 (90%) | 24 (92%) | 16 (89%) | χ2 = 0.18 (p = 0.92) |
| Time since injury (weeks) | – | 13.3 (5) | 13 (5.1) | F = 0.04 (p = 0.84) |
| Worst GCS score | – | 8.7 (4.6) | 7.4 (4) | F = 0.97 (p = 0.33) |
| Parent education (years) | 15.6 (3) | 13.7 (3.6) | 13 (3.8) | F = 4.47 (p = 0.02), 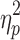 = 0.10 = 0.10 |
Worst GCS score = worst Glasgow Coma Scale score recorded in 24 h after injury. IHTT = interhemispheric transfer time averaged across left and right visual fields, in milliseconds. Parent education = highest number of years of education of either parent.
TBI, traumatic brain injury; SD, standard deviation.
Procedure
Neurocognitive tests
A cognitive performance composite index was computed that summarized performance on five tests that tapped the cognitive domains most sensitive to the effects of moderate and severe pediatric TBI in a recent meta-analytic review.29 The tests included in the cognitive composite index are briefly described below. Principal components analysis confirmed that these five tests shared sufficient common variance in both children with msTBI and healthy controls that they could be combined into a composite score with unit weighting (Moran and colleagues, 2015, submitted for publication). The five tests included the following:
Psychomotor processing speed
The Processing Speed Index (PSI) score from the Wechsler Intelligence Scale for Children-Fourth Edition or the Wechsler Adult Intelligence Scale-Third Edition, depending on age, was used.30,31
Working memory
The Working Memory Index score from the Wechsler Intelligence Scale for Children-Fourth Edition or WAIS-Third Edition, depending on age, was used.30,31
Verbal learning
The index of verbal learning was T-scores for the total words learned over five trials of the California Verbal Learning Test Children's Version (CVLT-C) or Second Edition (CVLT-II), depending on age.32,33
Executive functioning
The index of executive function was derived from the Inhibition/Switching condition of the Color-Word Interference subtest from the Delis-Kaplan Executive Functioning System battery.34
IQ estimate
The Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI)35 were used to generate a two subtest estimate of Full Scale IQ for all participants.
Visual ERP measure of IHTT
This study used visual ERPs to measure IHTT. IHTT is defined as the time required to transfer stimulus-locked neural activity between the left and right brain hemispheres. Electroencephalography was recorded while participants completed a computerized, pattern matching task with bilateral field advantage. A BIOSEMI system (BioSemi, Amsterdam, the Netherlands) was used to acquire ERPs. The low pass filter = 40 Hz, high pass filter = 0.16 Hz, bandwidth (3dB) = 134 Hz, and sample rate = 512 Hz.
Participants were exposed to two distinct visual patterns (geometric shapes of nine to 11 letter o's) following a gaze-fixation (a colon) in the center of the visual field. These patterns were presented randomly to two of the four visual fields (upper and lower; left and right). This created four bilateral and two unilateral conditions (right and left visual fields; RVF and LVF). Participants were asked to determine if the two patterns presented during each trial constituted a “match” or a “non-match”. “Match” and “non-match” responses were made by pressing the “M” and “N” keys of a computer keyboard. Participants alternated between pressing with the right hand with their index finger on the “N” key and their middle finger on the “M” key, or with the left hand with their index finger on the “M” key and middle finger on the “N” key for each trial. The responding hand was alternated in eight blocks of 97 trials. Each participant's cross-callosal IHTT was calculated using the electroencephalographic visual ERPs, which were collected during the unilateral conditions. Greater detail on this methodology is described elsewhere.36
ERP recording
While participants performed the pattern matching task, visual ERPs were recorded, synchronized to the onset of the pattern presentation. A 16-channel cap plus two grounders were used to record EEG. Parietal and occipital electrode sites were used because previous studies have shown these lateral sites produce large visual ERPs, which yield clear evoked potential IHTTs.37–39 In addition, electrodes were placed above, below, and at the outer cantus of each eye for the recording of eye movements. Electrodes placed on the mastoid bones (i.e., behind the ears) of participants were used as linked-ears references. This reference point has been shown to provide a more valid estimate of IHTT than mid-frontal reference points.28 ERPs at each recording electrode from each trial were stored on a computer disk for later averaging.
For each electrode, ERPs were averaged for the 2 × 2 combinations of LVF versus RVF. Averaged ERPs were displayed on a computer visual display and the N1 component identified blind to participant group. For each of the parietal or occipital recording electrodes—contingent upon which set provided the most clearly identifiable nodes and peaks—the latency and amplitude of these components were stored in a computer file for statistical analysis. IHTT was calculated by averaging ERP waveforms at the P3 or O1 (left hemisphere) and P4 or O2 (right hemisphere) electrode sites. Next, the peak latency (in milliseconds) of the early N1 evoked potential components was determined. Then, the latencies of the ipsilateral and contralateral conditions were subtracted to determine the overall IHTT for each visual field. Finally, the RVF and LVF IHTTs were averaged to compute the overall IHTT for each participant. The average of left to right visual field and right to left visual field IHTTs for each participant were used in the remaining analyses. Longer IHTTs indicate slower transfer of visual information across the posterior brain regions. Accuracy of pattern matching was not recorded in this study. Previous studies have demonstrated that true deficits in IHTT are reflected in slower reaction times rather than response accuracy, particularly in individuals with agenesis of the corpus callosum.40,41
Statistical analysis
Analyses reported include independent samples t-tests, Pearson correlations, analysis of covariance (ANCOVA), and univariate analysis of variance (ANOVA) tests. Data were analyzed using statistics program R and in SPSS v21 (IBM, Inc., Armonk, NY).
Results
IHTTs
The TBI group (mean = 15.5 msec, SD = 10.4) had a significantly slower IHTT than the control group (mean = 9.4 msec; SD = 5.6; F[1,83] = 10.5; p = 0.002; 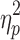 = 0.12). As noted above, given the heterogeneity of IHTT scores in the TBI group, the TBI group was divided into: 1) a “normal IHTT” TBI group and, 2) a second “slow IHTT” TBI group for further data analysis. The cutoff for the “slow IHTT” TBI group was an IHTT of 18ms or greater (I think I mentioned above, but how was the cutoff determined, a SD below control's mean?). The mean IHTT for the normal IHHT TBI, slow IHTT TBI, and control groups are presented in Table 1. As would be expected, the slow IHTT TBI group had a significantly slower IHHT than the control group and the normal IHTT TBI group (F[1,83] = 64.8; p < 0.001;
= 0.12). As noted above, given the heterogeneity of IHTT scores in the TBI group, the TBI group was divided into: 1) a “normal IHTT” TBI group and, 2) a second “slow IHTT” TBI group for further data analysis. The cutoff for the “slow IHTT” TBI group was an IHTT of 18ms or greater (I think I mentioned above, but how was the cutoff determined, a SD below control's mean?). The mean IHTT for the normal IHHT TBI, slow IHTT TBI, and control groups are presented in Table 1. As would be expected, the slow IHTT TBI group had a significantly slower IHHT than the control group and the normal IHTT TBI group (F[1,83] = 64.8; p < 0.001; 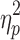 = 0.62).
= 0.62).
Parent education
There is a wide range of pre-injury cognitive functioning in children who incur TBIs. Since pre-injury cognitive function accounts for substantial variance in post-injury cognitive outcomes, it is important to control for the effects of pre-injury level of cognitive function. Parental level of education is a good predictor of a child's cognitive functioning42,43 and can therefore be used to control for what the child's level of cognitive function was absent the TBI. Parental education level was self-reported by parents. Parental education was missing for one participant in the control group. In instances where parents differed in their educational level, the higher educational level was selected.
The parental education of the slow IHTT TBI group was significantly lower than that of controls (F[2,82] = 4.5; p = 0.02; 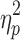 = 0.10), and was non-significantly lower than the normal IHTT TBI group. The healthy controls and normal IHTT TBI group did not significantly differ on parent education, although the mean parent education was somewhat lower for the normal IHTT TBI group than controls (see Table 1). Pearson correlations were performed to examine the relationship between parent education and IQ for study participants, by group. This analysis revealed that parent education was not significantly correlated with IQ for the normal IHTT TBI group (r = 0.28, p = 0.17). However, parent education was significantly correlated with IQ for both the slow IHTT TBI group (r = 0.57, p = 0.01) and for the healthy control group (r = 0.56, p < 0.001). As a consequence, parent education was used as a covariate for secondary analysis of the neurocognitive variable in this study to further evaluate differences between the three groups.
= 0.10), and was non-significantly lower than the normal IHTT TBI group. The healthy controls and normal IHTT TBI group did not significantly differ on parent education, although the mean parent education was somewhat lower for the normal IHTT TBI group than controls (see Table 1). Pearson correlations were performed to examine the relationship between parent education and IQ for study participants, by group. This analysis revealed that parent education was not significantly correlated with IQ for the normal IHTT TBI group (r = 0.28, p = 0.17). However, parent education was significantly correlated with IQ for both the slow IHTT TBI group (r = 0.57, p = 0.01) and for the healthy control group (r = 0.56, p < 0.001). As a consequence, parent education was used as a covariate for secondary analysis of the neurocognitive variable in this study to further evaluate differences between the three groups.
Neurocognitive test performance
The performance of the two TBI groups was compared to the healthy control children on the cognitive composite index score and IQ using 3 × 1 ANOVAs with the Bonferroni statistic used for post hoc comparisons. When there were group differences, the initial analyses were followed up with ANCOVAs with parental education as the covariate to correct for potential differences in pre-injury neurocognitive function. Cognitive composite index scores were not available for two normal IHTT TBI participants due to missing data in more than one neurocognitive subtest for these participants (e.g., no PSI subtests taken due to participants' physical motor impairments). The results of comparisons between the remaining participants are summarized in Table 2.
Table 2.
IHTT and Neurocognitive Scores for Controls and Two TBI Groups
| Control Mean (SD) | Normal IHTT TBI Mean (SD) | Slow IHTT TBI Mean (SD) | F value (and significance) | F value with parent education covariate (and significance) | |
|---|---|---|---|---|---|
| N | 39 | 26 | 18 | ||
| IHTT Total | 9.4 (5.6) | 8 (5.1) | 25.9 (6.4)* | F = 64.8 (p < 0.001), 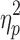 = 0.62 = 0.62 |
F = 61.7 (p < 0.001), 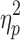 = 0.61 = 0.61 |
| IHTT LVF | 9.3 (7.2) | 8.4 (6.4) | 24 (11.0)* | F = 24.7 (p < 0.001), 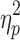 = 0.39 = 0.39 |
F = 22.6 (p < 0.001), 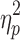 = 0.38 = 0.38 |
| IHTT RVF | 9.4 (6.9) | 8.5 (6.4) | 27.8 (11.2)* | F = 38.9 (p < 0.001), 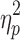 = 0.51 = 0.51 |
F = 37.4 (p < 0.001), 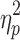 = 0.50 = 0.50 |
| Neurocognitive Performance Index | 103.4 (10.8) | 98.4 (10.7) (n = 24) | 90.3 (13.5)* | F = 7.9 ( p = 0.001), 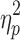 = 0.17 = 0.17 |
F = 5.2 (p = 0.008), 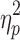 = 0.12 = 0.12 |
Slow IHTT TBI group significantly different from controls; normal IHTT TBI group not significantly different.
IHTT = interhemispheric transfer time averaged across left and right visual fields, in milliseconds; IHTT LVF = time for stimuli unilaterally presented to left visual field to transfer from the right to left hemisphere, in milliseconds; IHTT RVF = time for stimuli unilaterally presented to right visual field to transfer from left to right hemisphere, in milliseconds.
TBI, traumatic brain injury; SD, standard deviation.
Cognitive composite index
There was a significant difference between the three groups on the cognitive composite index (F[1,81] = 7.9; p = 0.001; 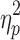 = 0.17). Post hoc comparisons revealed that the slow IHTT TBI group had significantly poorer neurocognitive performance than the control group. There were no statistically significant differences in the cognitive composite index between the normal IHTT TBI group and the healthy control group nor between the two TBI groups. The observed differences between the healthy control and slow IHTT TBI groups survived correction for parental education (F[1,80] = 5.2; p = 0.008;
= 0.17). Post hoc comparisons revealed that the slow IHTT TBI group had significantly poorer neurocognitive performance than the control group. There were no statistically significant differences in the cognitive composite index between the normal IHTT TBI group and the healthy control group nor between the two TBI groups. The observed differences between the healthy control and slow IHTT TBI groups survived correction for parental education (F[1,80] = 5.2; p = 0.008; 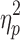 = 0.12). A secondary, pairwise comparison (i.e., 2 × 1 ANOVA of controls and the slow IHTT TBI group) produced similar results (F[1,56] = 8.1; p = 0.006;
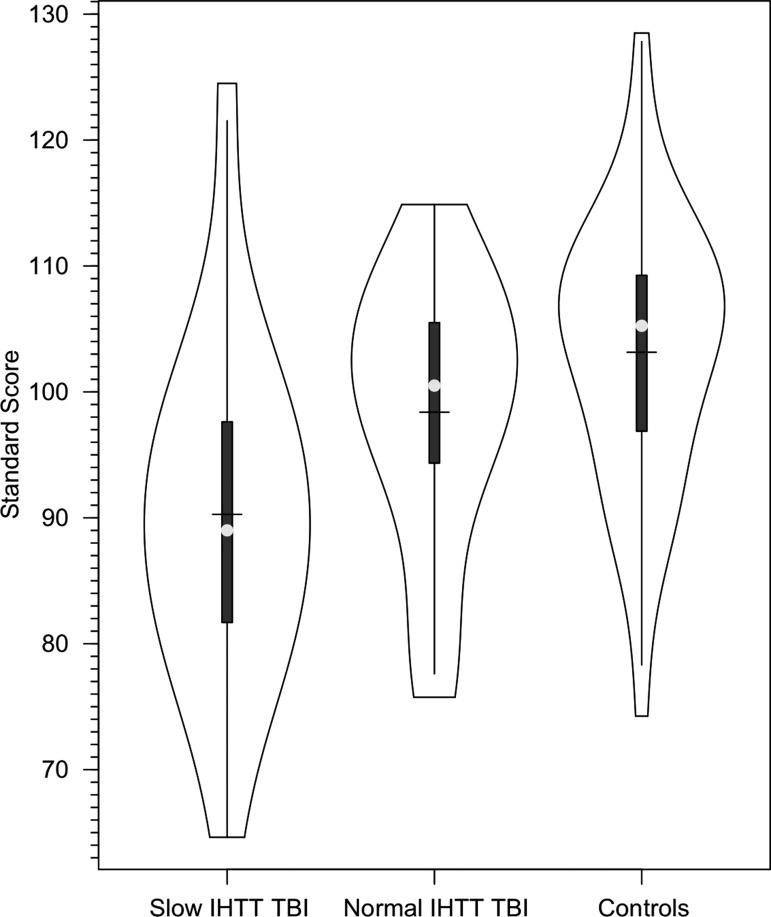
= 0.12). A secondary, pairwise comparison (i.e., 2 × 1 ANOVA of controls and the slow IHTT TBI group) produced similar results (F[1,56] = 8.1; p = 0.006; 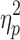 = 0.13). The relation between IHTT and the cognitive composite index scores was not linear in patients with TBI. The correlation between IHTT and cognitive composite index scores in the overall TBI group was not significant. Figure 2 presents the distribution of the cognitive composite index scores in the three groups.
= 0.13). The relation between IHTT and the cognitive composite index scores was not linear in patients with TBI. The correlation between IHTT and cognitive composite index scores in the overall TBI group was not significant. Figure 2 presents the distribution of the cognitive composite index scores in the three groups.
FIG. 2.
Violin plots of the distribution of Cognitive Composite Index Scores in the healthy control, slow, and normal interhemispheric transfer time (IHTT) moderate-to-severe traumatic brain injury groups.
Two-subtest IQ
Initially, there was a significant difference in the two-subtest IQs of the three groups (F[1,83] = 4.3; p = 0.02; 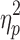 = 0.10). The slow IHTT TBI group had a significantly lower IQ than controls (p = 0.01), while the difference between controls and the normal IHTT TBI group did not reach significance (p = 0.90), and similarly, there was no significant difference between the two TBI groups (p = 0.20). However, when parent education was corrected for, there were no significant differences between the three groups (p = 0.14). As noted previously, IQ was significantly correlated with parent education for the healthy control and slow IHTT TBI groups, but not for the normal IHTT TBI group.
= 0.10). The slow IHTT TBI group had a significantly lower IQ than controls (p = 0.01), while the difference between controls and the normal IHTT TBI group did not reach significance (p = 0.90), and similarly, there was no significant difference between the two TBI groups (p = 0.20). However, when parent education was corrected for, there were no significant differences between the three groups (p = 0.14). As noted previously, IQ was significantly correlated with parent education for the healthy control and slow IHTT TBI groups, but not for the normal IHTT TBI group.
Discussion
Children with msTBIs were studied on average within 3 months post-TBI. Overall, the TBI group had significantly longer IHTTs than the healthy control group. Just under half of the children with TBIs had IHTT scores outside the normal range (i.e., more than 1.5 standard deviations longer than the mean IHTT score of the healthy control group). The slow IHTT TBI group had impaired interhemispheric communication. This finding is consistent with the results of two prior studies, which used a verbal dichotic listening task in children with severe TBIs10 as well as visual and tactile reaction time tasks in adults with TBI20 to assess interhemispheric communication. Because the ERP IHTT task is an electrophysiological measure of IHTT, it provides a more direct indication of the functional integrity of the CC than performance based measures. By providing a relatively direct measure of the functional integrity of the CC, the ERP IHTT is a very useful complement to the increasingly sensitive MRI measures, such as high resolution DTI, used to assess the structural integrity of the CC.
In children with TBI, the slow IHTT group, but not the normal IHTT group, had neurocognitive impairments. After correcting for parental education (to control for pre-injury level of cognitive function), the slow IHTT TBI group performed significantly more poorly than healthy controls on the cognitive composite index score. While the normal IHTT TBI group performed more poorly than the healthy control group on this index score, the differences between the normal IHTT TBI group and the control group did not reach significance. The slow and normal IHTT TBI groups did not significantly differ from each other in neurocognitive functioning, although the slow IHTT TBI group tended to have lower scores on the cognitive composite index score than the normal IHTT TBI group. The worst neurocognitive performance in the current study was found in children who had both incurred a TBI and had slow IHTT. A slow IHTT following a msTBI captures some, but not all, of the variance in the adverse effect of a TBI on neurocognitive functioning.
There are two non-mutually exclusive hypotheses about the mechanisms underlying the effect of slow IHTT on neurocognitive function following TBI. Both explanations assume that slow IHTT following a TBI reflects decreased functional integrity of the CC. The first explanation is that many higher order cognitive processes (i.e., particularly those that are computationally demanding) involve the coordinated activity of processing nodes in both hemispheres.44 Damage to the CC disrupts interhemispheric collaboration.
The second hypothesis stems from the observation that, given the diffuse damage to white matter tracts caused by moderate and severe TBIs, white matter damage is not restricted to the CC. The disruption to the functional integrity of the CC reflected in slow IHTT in many, but not all, children with TBI may be a reflection of more general damage to white matter tracts. In effect, the disruption of the functional integrity of the CC may be a specific instance of a more general problem.
Conclusions and Directions for Future Research
This study was the first to examine IHTT in children at a circumscribed time-point during the first year post-TBI—about 3 months post-injury. This paper does not address the question of the course of IHTT after the post-acute phase, nor how well IHTT predicts longer-term outcomes. When more longitudinal data is available, we will determine whether IHTT normalizes during the first year post-TBI and whether normalization of IHTT predicts neurocognitive function during this time frame. Future research should examine the correlation between IHTT and structural indices of white matter damage. When more brain imaging data is available, we will examine the relationship between measures of white matter damage (including high resolution DTI) in the CC and long projection tracts outside of the CC and IHTT using this ERP method. This is one way to address the second hypothesis. In a very small subset (n = 4) of the patients in the current study studied post-acutely, we found that IHTT measured by EEG-ERP was inversely and significantly correlated with posterior CC N-acetyl acetate levels (neuronal/axonal integrity) and positively correlated with posterior CC choline (membrane degeneration/inflammation) and creatine (energy metabolism) levels.3 We will determine if these results can be replicated in a larger sample.
Acknowledgements
This research was supported by National Institute of Child Health and Human Development Grant #HD061504. We thank Alma Martinez and Alma Ramirez for their assistance in data collection and the children and their parents who participated in this research.
Author Disclosures Statement
No competing financial interests exist.
References
- 1.Levin H.S., Hanten G., Roberson G., Li X., Ewing-Cobbs L., Dennis M., Chapman S., Max J.E., Hunter J., Schachar R., Luerssen T.G., and Swank P. (2008). Prediction of cognitive sequelae based on abnormal computed tomography. J. Neurosurg. Pediatr. 1, 461–470 [DOI] [PubMed] [Google Scholar]
- 2.Reeves T. M., Phillips L. L., and Povlishock J. T. (2005). Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp. Neurol. 196, 126–137 [DOI] [PubMed] [Google Scholar]
- 3.Babikian T., Marion S. D., Copeland S., Alger J. R., O'Neill J., Cazalis F., Mink R., Giza C. C., Vu J. A., Hilleary S. M., Kernan C. L., Newman N., and Asarnow R. (2010). Metabolic levels in the corpus callosum and their structural and behavioral correlates after moderate to severe pediatric TBI. J. Neurotrauma 27, 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ljungqvist J., Nilsson D., Ljungberg M., Sörbo A., Esbjörnsson E., Eriksson-Ritzén C., and Skoglund T. (2011). Longitudinal study of the diffusion tensor imaging properties of the corpus callosum in acute and chronic diffuse axonal injury. Brain Inj. 25, 370–378 [DOI] [PubMed] [Google Scholar]
- 5.Wilde E. A., Chu Z., Bigler E. D., Hunter J. V., Fearing M. A., Hanten G., Newsome M. R., Scheibel R. S., Li X., and Levin H. S. (2006). Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J. Neurotrauma 23, 1412–1426 [DOI] [PubMed] [Google Scholar]
- 6.Wozniak J. R., Krach L., Ward E., Mueller B. A., Muetzel R., Schnoebelen S., Kiragu A., and Lim K. O. (2007). Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch. Clin. Neuropsychol. 22, 555–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu T. C., Wilde E. A., Bigler E. D., Li X., Merkley T. L., Yallampalli R., McCauley S. R., Schnelle K. P., Vasquez A. C., Chu Z., Hanten G., Hunter J. V., and Levin H. S. (2010). Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Dev. Neurosci. 32, 361–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin H. S., Benavidez D., Verger-Maestre K., Perachio N., Song J., Mendelsohn D., and Fletcher J. M. (2000). Reduction of corpus callosum growth after severe traumatic brain injury in children. Neurology 54, 647–653 [DOI] [PubMed] [Google Scholar]
- 9.Johnson S. C., Pinkston J. B., Bigler E. D., and Blatter D. D. (1996). Corpus callosum morphology in normal controls and traumatic brain injury: sex differences, mechanisms of injury, and neuropsychological correlates. Neuropsychology 10, 408–415 [Google Scholar]
- 10.Benavidez D. A., Fletcher J. M., Hannay J. H., Bland S. T., Caudle S. E., Mendelsohn D., Yeakley J., Brunder D. G., Howard H., Song J., Perachio N. A., Bruce D., Scheibel R. S., Lilly M.A., Verger-Maestre K., and Levin H. S. (1999). Corpus callosum damage and interhemispheric transfer of information following closed head injury in children. Cortex 35, 315–336 [DOI] [PubMed] [Google Scholar]
- 11.Gentry L. R., Thompson B., and Godersky J. C. (1988). Trauma to the corpus callosum: MR features. Am J. Neuroradiol. 9, 1129–1138 [PMC free article] [PubMed] [Google Scholar]
- 12.Lindenberg R., Fisher R. S., Durlacher S. H., Lovitt W. V., and Freytag E. (1955). Lesions of the corpus callosum following blunt mechanical trauma to the head. Am J. Pathol. 31, 297–317 [PMC free article] [PubMed] [Google Scholar]
- 13.Mendelsohn D. B., Levin H. S., Harward H., and Bruce D. (1992). Corpus callosum lesions after closed head injury in children: MRI, clinical features and outcome. Neuroradiology 34, 384–388 [DOI] [PubMed] [Google Scholar]
- 14.Peru A., Beltramello A., Moro V., Sattibaldi L., and Berlucchi G. (2003). Temporary and permanent signs of interhemispheric disconnection after traumatic brain injury. Neuropsychologia 41, 634–643 [DOI] [PubMed] [Google Scholar]
- 15.Shiramizu H., Masuuko A., Ishizaka H., Shibata M., Atsumi H., Imai M., Osada T., Mizokami Y., Baba T., and Matsumae M. (2008). Mechanism of injury to the corpus callosum, with particular reference to the anatomical relationship between site of injury and adjacent brain structures. Neurol. Med. Chir. (Tokyo) 48, 1–7 [DOI] [PubMed] [Google Scholar]
- 16.Vuilleumier P. and Assal G. (1995). Complete callosal disconnection after closed head injury. Clin. Neurol. Neurosurg. 97, 39–46 [DOI] [PubMed] [Google Scholar]
- 17.Slawik H., Salmond C. H., Taylor-Tavares J. V., Williams G. B., Sahakian B. J., and Tasker R. C. (2009). Frontal cerebral vulnerability and executive deficits from raised intracranial pressure on child traumatic brain injury. J. Neurotrauma 26, 1891–1903 [DOI] [PubMed] [Google Scholar]
- 18.Tasker R. C., Westland A. G., White D. K., and Williams G. B. (2010). Corpus callosum and inferior forebrain white matter microstructure are related to functional outcome from raised intracranial pressure in child traumatic brain injury. Dev. Neurosci. 32, 374–384 [DOI] [PubMed] [Google Scholar]
- 19.Brown W. S., Jeeves M. A., Deitrich R., and Burnison D. S. (1999). Bilateral field advantage and evoked potential interhemispheric transmission in commisurotomy and callosal agenesis. Neuropsychologia 37, 1165–1180 [DOI] [PubMed] [Google Scholar]
- 20.Rugg M. D., Milner A. D., and Lines C. R. (1985). Visual evoked potentials to lateralised stimuli in two cases of callosal agenesis. J. Neurol. Neurosurg. Psychiatry 48, 367–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peru A., Beltramello A., Moro V., Sattibaldi L., and Berlucchi G. (2003). Temporary and permanent signs of interhemispheric disconnection after traumatic brain injury. Neuropsychologia 41, 634–643 [DOI] [PubMed] [Google Scholar]
- 22.Damasio A. R., Chui H. C., Corbett J., and Kassel N. (1980). Posterior callosal section in a non-epileptic patient. J. Neurol. Neurosurg. Psychiatry 43, 351–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gazzaniga M. S. and Freedman H. (1973). Observations on visual processes after posterior callosal section. Neurology 23, 1126–1130 [DOI] [PubMed] [Google Scholar]
- 24.Sugishita M. and Yoshioka M. (1987). Visual processes in a hemialexic patient with posterior callosal section. Neuropsychologia 25, 329–339 [DOI] [PubMed] [Google Scholar]
- 25.Mathias J. L., Bigler E. D., Jones N. R., Bowden S. C., Barrett-Woodbridge M., Brown G. C., and Taylor D. J. (2004). Neuropsychological and information processing performance and its relationship to white matter changes following moderate and severe traumatic brain injury: a preliminary study. Appl. Neuropsychol. 11, 134–152 [DOI] [PubMed] [Google Scholar]
- 26.Kumar R., Gupta R. K., Husain M., Chaudhry C., Srivastava A., Saksena S., and Rathore R. K. (2009). Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: Its correlation with neuropsychometric tests. Brain Inj. 23, 675–685 [DOI] [PubMed] [Google Scholar]
- 27.Verger K., Junque C., Levin H. S., Jurado M. A., Perez-Gomez M., Bartres-Faz D., Barrios M., Alvarez A., Bartumeus F., and Mercader J. M. (2001). Correlation of atrophy measures on MRI with neuropsychological sequelae in children and adolescents with traumatic brain injury. Brain Inj. 15, 211–221 [DOI] [PubMed] [Google Scholar]
- 28.Saron C. D. and Davidson R. J. (1989). Visual evoked potential measures of interhemispheric transfer time in humans. Behav. Neurosci. 103, 1115–1138 [DOI] [PubMed] [Google Scholar]
- 29.Babikian T. and Asarnow R. (2009). Neurocognitive outcomes and recovery after pediatric TBI: meta-analytic review of the literature. Neuropsychology 23, 283–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wechsler D. (1997). Wechsler Adult Intelligence Scale–Third Edition (WAIS-III). The Psychological Corporation: San Antonio, TX [Google Scholar]
- 31.Wechsler D. (2003). Wechsler Intelligence Scale for Children- Fourth Edition (WISC-IV). The Psychological Corporation: San Antonio, TX [Google Scholar]
- 32.Delis D., Kramer J. H., Kaplan E., and Ober B. A. (1994). California Verbal Learning Test–Children's Version (CVLT-C). The Psychological Corporation: San Antonio, TX [Google Scholar]
- 33.Delis D., Kramer J. H., Kaplan E., and Ober B. A. (2000). California Verbal Learning Test–Second Edition (CVLT-II). The Psychological Corporation: San Antonio, TX [Google Scholar]
- 34.Delis D., Kaplan E., and Kramer J. H. (2001). Delis-Kaplan Executive Function System (D-KEFS). The Psychological Corporation: San Antonio, TX [Google Scholar]
- 35.Wechsler D. (1999). Wechsler Abbreviated Intelligence Scale (WASI). The Psychological Corporation: San Antonio, TX [Google Scholar]
- 36.Larson E. B. and Brown W. (1997). Bilateral field interactions, hemispheric specialization and evoked potential interhemispheric transmission time. Neuropsychologia 35, 573–581 [DOI] [PubMed] [Google Scholar]
- 37.Andreassi J. L., Okamura H., and Stern M. (1975). Hemispheric asymmetries in the visual cortical evoked potential as a function of stimulus location. Psychophysiology 12, 541–546 [DOI] [PubMed] [Google Scholar]
- 38.Brown W. S. and Jeeves M. A. (1993). Bilateral visual filed advantage and evoked potential interhemispheric trasmission time. Neuropsychologia 31, 1267–1281 [DOI] [PubMed] [Google Scholar]
- 39.Ledlow A., Swanson J. M., and Kinsbourne M. (1978). Differences in reaction times and average evoked potentials. Ann. Neurol. 3, 525–530 [DOI] [PubMed] [Google Scholar]
- 40.Lassonde M., Sauerwein H. C., McCabe N., Laurencelle L., and Geoffroy G. (1988). Extent and limits of cerebral adjustment to early section or congenital absence of the corpus callosum. Behav. Brain Res. 30, 165–181 [DOI] [PubMed] [Google Scholar]
- 41.Sauerwein H. C. and Lassonde M. (1983). Intra- and interhemispheric processing of visual information in callosal agenesis. Neuropsychologia 21, 167–171 [DOI] [PubMed] [Google Scholar]
- 42.Buckhalt J. A., El-Sheikh M., Keller P. S., and Kelly R. J. (2009). Concurrent and longitudinal relations between children's sleep and cognitive functioning: the moderating role of parent education. Child Dev. 80, 875–892 [DOI] [PubMed] [Google Scholar]
- 43.Davis-Kean P. E. (2005). The influence of parent education and family income on child achievement: the indirect role of parental expectations and the home environment. J. Fam. Psychol. 19, 294–304 [DOI] [PubMed] [Google Scholar]
- 44.Banich M. T. and Belger A. (1990). Interhemispheric interaction: how do the hemispheres divide and conquer a task? Cortex 26, 77–94 [DOI] [PubMed] [Google Scholar]