Table 1.
Physico-chemical characteristics of aminomethyl derivatives of benzothiazol- and benzoxazolthiones
| Found/calculated (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Compound | The melting point (°C) | Yield (%) | C | H | N | S | Brutto formula | NMR spectra δ (ppm) |
| 1 | 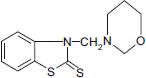 |
120–121 | 45 | 54.61 54.13 | 5.76 5.26 | 10.60 10.52 | 24.20 24.06 | C12H14N2OS2 | 1.79 (kv. 2H, CH2CH2CH2), 3.29(t., 2H, NCH2), 3.9 (t., 2H, ОCH2), 4.567 (t., 2H, NCH2N), 5.3 (s., 2H, NCH2O), 6.8–7.6 (m., 4H, C6H4). |
| 2 | 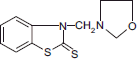 |
124–126 | 40 | 56.00 57.60 | 5.90 5.30 | 10.30 10.50 | 26.60 24.60 | C11H12N2OS2 | 1.819 (kv. 2H, CH2CH2CH2), 3.119 (t., 2H, NCH2), 3.9 (t., 2H, ОCH2), 4.567 (t., 2H, NCH2N), 5.3 s. 2H (NCH2O), 7.1–7.3 m. 4H (C6H4) |
| 3 | 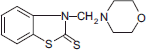 |
134 | 40 | 54.9 54.13 | 5.55 5.26 | 11.01 10.52 | 24.56 24.06 | C12H14N2OS2 | 1.7 (m., 2H, CH2CH2CH2), 1.97 (m., 4H, CH2CH2CH2), 3.01 (t., 4H, NCH2N), 7.6–8.9 (d., 4H, C5-C7), 8.1 (s., 2H, ArCH2N). |
| 4 | 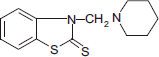 |
152–152.5 | 42 | 57.6 57.0 | 7.5 5.6 | 11.3 11.2 | 28.8 25.6 | C13H16N2S2 | 1.5 (m., 2H, CH2CH2CH2), 1.7 (m., 4H, CH2CH2CH2), 3.15 (t., 4H, NCH2N), 7.1–7.6 (d., 4H, C5-C7), 8 (s., 2H, ArCH2N). |
| 5 | 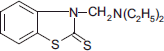 |
128–130 | 35 | 54.4 57.14 | 5.98 6.35 | 8.2 11.11 | 27.0 25.4 | C12H16N2S2 | 2.19 (kv, 2H, CH2CH2CH2), 3.12 (t., 2H, NCH2), 3.6 (t., 2H, ОCH2), 4.67 (t., 2H, NCH2N), 5.20 s. 2H (NCH2O), 7.1–7.5 m. 4H (C6H4). |
| 6 | 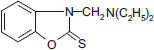 |
105 | 65 | 59.87 61.02 | 5.98 6.78 | 10.85 11.86 | 13.03 13.56 | C12H16N2OS | 1.28 (m., 2H, CH2CH2CH2), 3.04 (t., 2H, CH2N), 3.69 t., (2H, CH2О), 4.59 (s., 2H, NCH2N), 5.36 (s., 2H, NCH2O), 6.9–7.9 (m., 4H, C6H4). |
| 7 | 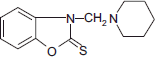 |
125 | 45 | 63.52 62.9 | 6.04 6.45 | 9.39 11.29 | 11.53 12.9 | C13H16N2OS | 1.88 (m., 2H, CH2CH2CH2), 3.18 (t., 2H, CH2N), 3.9 (t., 2H, CH2О), 4.39 (s., 2H, NCH2N), 5.56 (s. 2H, NCH2O), 7.4–7.7 (m., 4H, C6H4). |
| 8 | 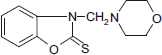 |
145–147 | 48 | 57.58 57.6 | 5.7 5.6 | 11.34 11.2 | 12.54 12.8 | C12H14N2O2S | 1.21 (d., 2H, CH2CH2CH2), 3.23 (t., 2H, CH2N), 3.7 (t., 2H, CH2О), 4.39 (s., 2H, NCH2N), 5.86 (s., 2H, NCH2O), 7.14–7.97 (m., 4H, C6H4). |
| 9 | 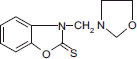 |
115–118 | 60 | 55.84 55.93 | 5.25 5.08 | 11.65 11.86 | 13.87 13.55 | C11H12N2O2S | 1.38 (m., 2H, CH2CH2CH2), 2.08 (t. 2H, CH2N), 3.39 (t., 2H, CH2О), 3.59 (s., 2H, NCH2N), 5.16 (s., 2H, NCH2O), 7.1–8.7 (m., 4H, C6H4). |
| 10 | 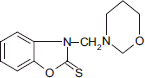 |
145–147 | 67.6 | 56.0 57.6 | 5.51 5.6 | 11.0 11.2 | 13.3 12.8 | C12H14N2O2S | 1.8 (m., 2H, CH2CH2CH2), 3.08 (t., 2H, CH2N), 3.9 (t., 2H, CH2О), 4.59 (s., 2H, NCH2N), 5.56 (s.,. 2H, NCH2O), 7.4–7.7 (m., 4H, C6H4). |
