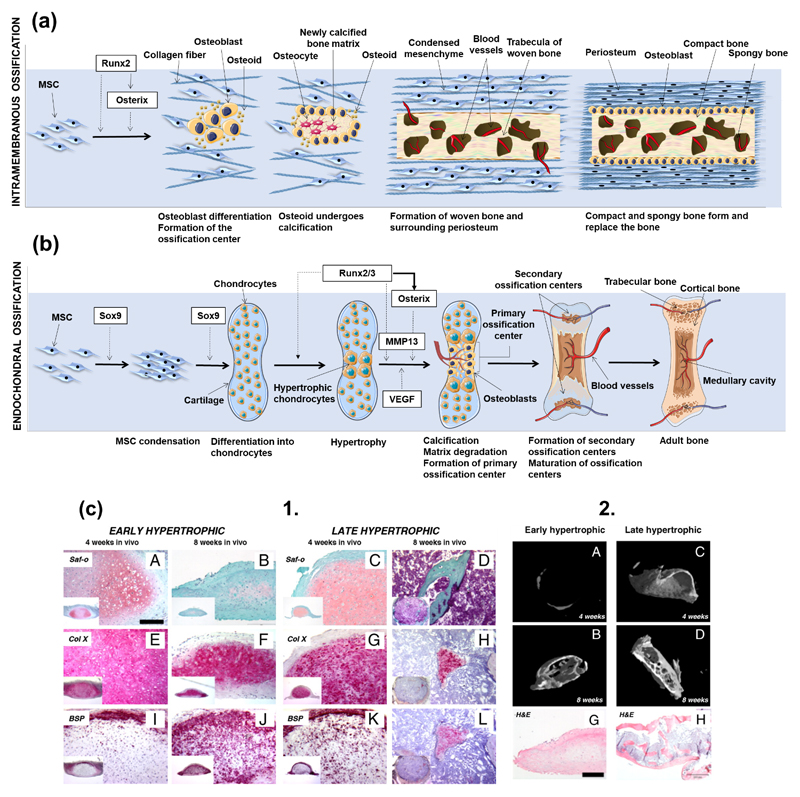
Figure 2.
(a) Schematic representation of intramembranous ossification. At an initial stage, MSCs cluster and differentiate into osteoblasts, forming the ossification center. Runx2 is deeply involved in the regulation of osteogenic differentiation, either directly or by inducing the late expression of Osterix. Osteoblasts start to produce the osteoid, which calcifies in few days. Osteoblasts trapped into the calcified matrix differentiate into osteocytes. Vascularized mesenchyme condenses on the external area of the woven bone, generating the periosteum. The woven bone is produced, with vascularized internal spaces that will form the marrow cavity. The surface of trabeculae is filled with matrix forming the compact bone. Spongy bone persists at the inner part. (b) Schematic representation of endochondral ossification. After condensation, MSCs starts to differentiate into chondrocytes, generating a cartilage template. Chondrocytes in the middle of the cartilage become hypertrophic. Sox9 and Runx2/3 are indispensable transcription factors for the initiation of chondrogenesis and the hypertrophy of chondrocytes, respectively. Hypertrophic chondrocytes induce vascular invasion. At this stage, Osterix functions as both a downstream and transcriptional partner of Runx2/3 during calcification and matrix degradation in cartilage, and cooperate with Runx2/3 to induce MMP13 expression. Osteoblasts differentiate from cells brought into the cartilage template with blood vessels invasion, starting to produce bone at a primary ossification center. Bone formation then spreads along the shaft forming secondary ossification centers. Finally, the adult bone, containing both trabecular and cortical bones and the medullary cavity is formed. (c) 1. Scotti et al. [74] induced endochondral bone formation in vitro using human MSCs. Hypertrophic tissue structures were implanted into nude mice to assess their ability to form trabecula bone. Both early (A-J) and later (C-L) hypertrophic samples went towards differentiation after in vivo implantation, although the latter specimen presented a more intense remodeling after 4 weeks (K), with the cartilaginous template almost resorbed after 8 weeks (L). (c) 2. Quantitative microtomography (μCT) of explants demonstrated that the deposition of mineralized matrix at the early hypertrophic samples (A-B) was reduced when compared to the late hypertrophic implanted structures (C-D). In fact, late hypertrophic samples displayed an interconnected network of trabeculae throughout the core after 8 weeks of implantation (D) [81]. Histological analysis by hematoxylin/eosin staining (G-H) revealed the presence of trabecular-like structures in the outer collar and inner core of the late, but not of the early, hypertrophic samples [81]. Figure 2(b) was adapted from Reference [81].