Fig. 1.
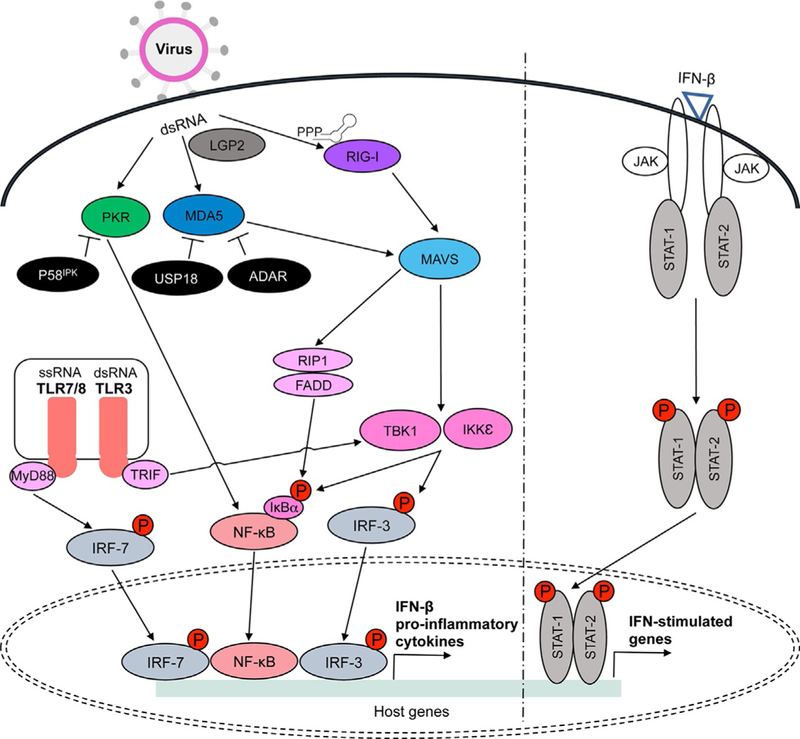
RIG-I, MDA5, LGP2, PKR, and TLRs3/7/8 are activated during viral infection in the presence of non-self dsRNA. RIG-I recognizes short dsRNA and non-self ssRNA with 5′-triphosphate or 5′-diphosphate, while MDA5 recognizes long dsRNA. LGP2 acts upstream to regulate RIG-I and MDA5 activation in the presence of dsRNA. RIG-I and MDA5 interact with MAVS adaptor protein located on the mitochondrial membrane. MAVS then activates FADD/RIP1 and TBK1/IKKε protein kinases that induce nuclear translocation of NF-κB and IRF3 following phosphorylation of IκBα or IRF3. PKR is also activated in the presence of dsRNA and it transmits activation signals through NF-κB. Endolysosomal TLRs3 and 7/8 lead to TBK1 and IRF7 activation via adaptors TRIF and MyD88, respectively. NF-κB, IRF3, and IRF7 are transcription factors that induce proinflammatory cytokine production, including IFN-β. Autoamplification of IFN signaling occurs through activation of the Jak/STAT pathway upon IFN-β binding. While IFN-stimulated genes spread antiviral signals to surrounding cells, viral proteins inhibit multiple steps of these pathways (see Table 1). Inhibitors of these signaling pathway also work to control activation signals and dysfunctions in these molecules can contribute to various autoimmune disorders. For example, P58IPK inhibits PKR while USP18 and ADAR suppress MDA5 activity (see Table 2).
