Fig 4. Binding of C1 and C1r to BBK32 orthologues via far western blot analysis.
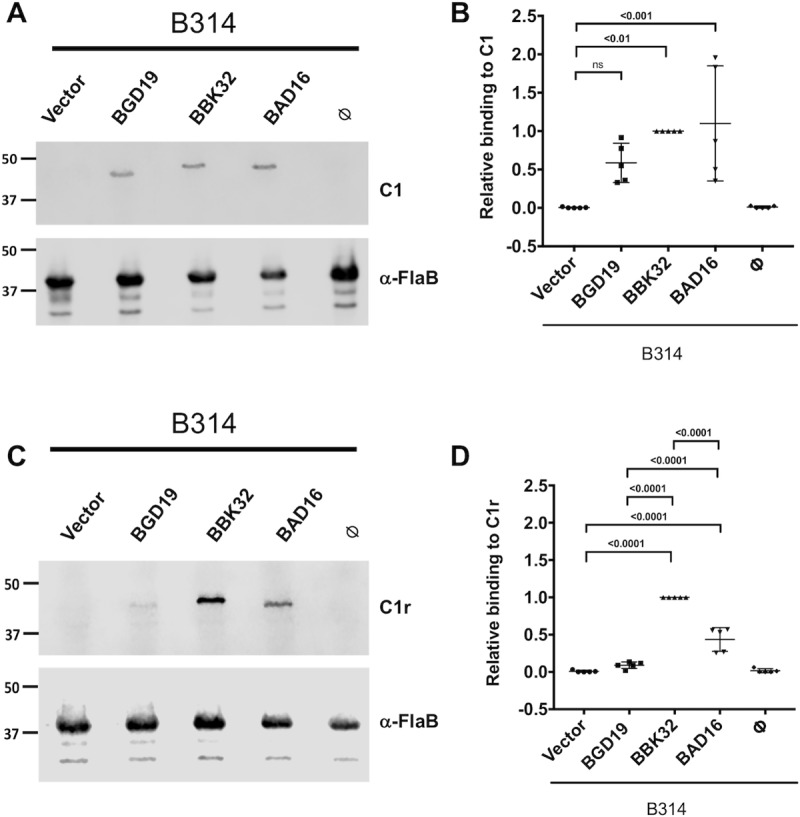
A-D) BGD19, BBK32, and BAD16 were expressed as lipoproteins on the surface of B. burgdorferi B314. Whole cell protein lysates were separated on an SDS-PAGE gel and probed for binding to human C1 (panel A) or C1r (panel C) using a Far Western blot overlay. Samples tested include strain B314/pBBE22luc (vector only control; labeled as “Vector”), B314/pBDG19 (labeled as BGD19), B314/pCD100 (labeled as BBK32), B314/pBAD16 (labeled as BAD16), and B314 alone (labeled as null). FlaB was used as a loading control to normalize variation between C1 and C1r binding by BBK32, BAD16, and BGD19 in panels A and C. Densitometry was performed from independent blots to quantify the observed signals as depicted in panels A and C. Panels B and D report the signal detected for C1 and C1r to the samples indicated on the x axis, respectively. All values were normalized relative to BBK32 binding to either C1 or C1r. P values between samples are indicated above the bars.
