Figure 2. 5F02 enhanced anti-CML effect of imatinib + talazoparib.
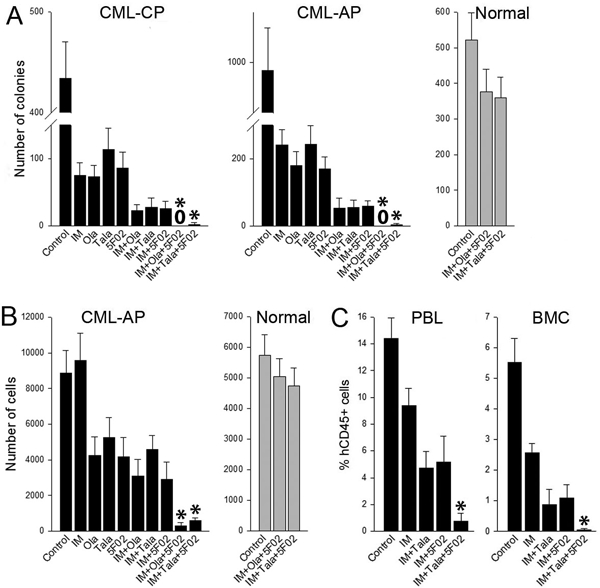
(A, B) Lin-CD34+ CML-CP/AP cells (black bars; n=3 patients of each) and normal counterparts (grey bars; n=3 healthy donors) were stained with cell trace violet (CTV) (eBioscience) and incubated for 5 days with 1μM imatinib (IM), 2.5μM 5F02, 2.5μM olaparib (Ola), 25nM talazoparib (Tala) or with indicated combinations in StemSpan®SFEM medium (Stem Cell Technologies, Vancouver, Canada) supplemented with the cocktail of growth factors (see Figure 1 legend). (A) Lin-CD34+ clonogenic cells were detected after plating in Methocult. (B) Lin-CD34+CD38-CTVmax cells were detected by flow cytometry using fluorochrome-conjugated anti-Lin (#340546), anti-CD34 (#347203, #555821) and anti-CD38 (#355790, #555460) antibodies (all from BD Pharmingen). Results represent mean number of colonies or quiescent cells ± SD from triplicates; *p<0.001 in comparison to IM+Ola, and IM+5F02 using the response additivity approach to study synergistic effects. (C) Sub-lethally total-body irradiated (600 Gy) NOD.Rag1−/−;γcnull mice expressing human IL-3, GM-CSF and SCF (NRGS) were injected with 107 Lin-CD34+ CML-CP cells and treated for 7 days with imatinib (IM) (100 mg/kg twice daily by oral gavage [6]) and indicated combinations with 5F02 (2.5 mg/kg i.p.) and/or talazoparib (Tala) (0.33 mg/kg i.v. [6]). Anti-CML effect was assessed by detection of hCD45+ cells in peripheral blood leukocytes (PBL) and bone marrow cells (BMC) at the end of treatment as described before [6]. Results represent mean ± SD percentage of hCD45+ in PBL and BMC (3–4 mice/group); *p<0.01 in comparison to IM+Tala, and IM+5F02 using the response additivity approach to study synergistic effects.
