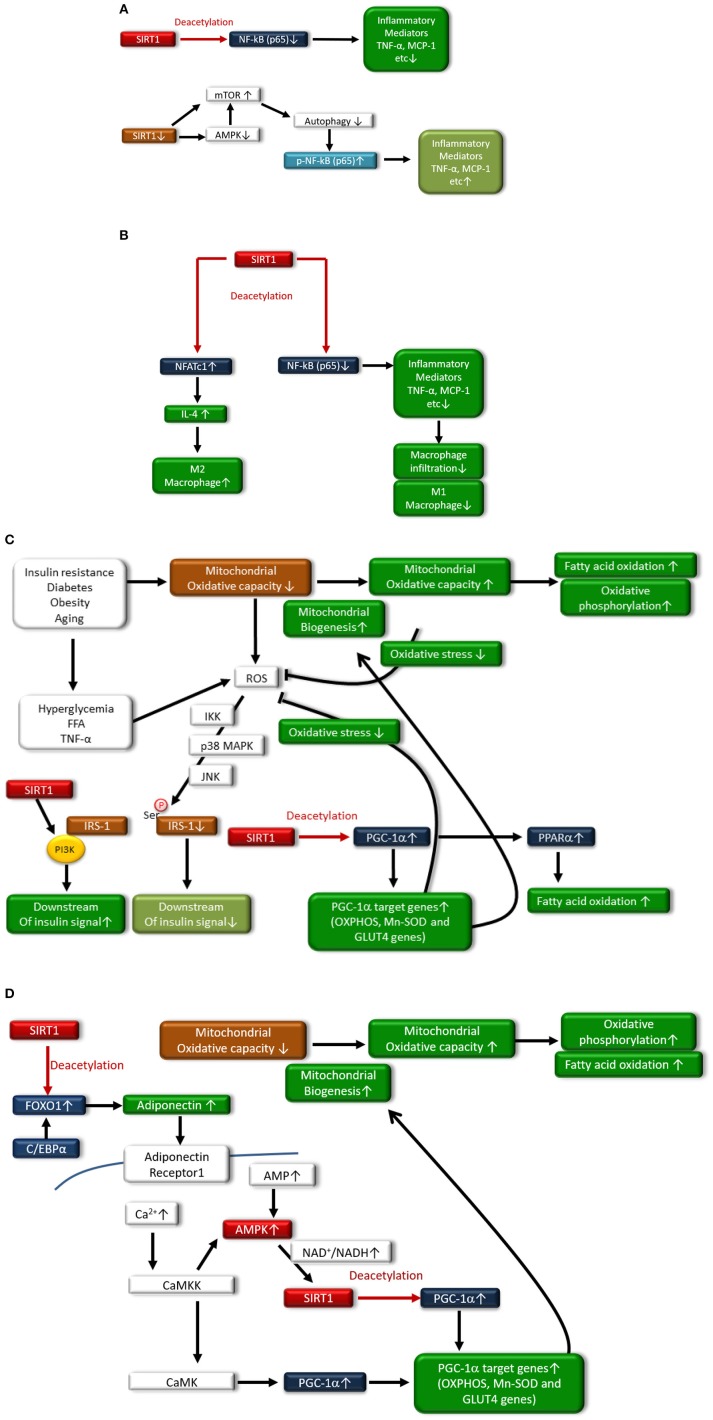
Figure 2.
(A) In monocytes/macrophages and adipocytes, SIRT1 deacetylates NF-κB(p65), resulting in reduced expression of inflammatory mediators such as TNF-α and MCP-1. SIRT1 inactivation also induces inflammation through the phosphorylation of the NF-κB pathway via impaired autophagy, which is associated with activation of mammalian target of rapamycin (mTOR) and reduced activation of AMP-activated kinase (AMPK). (B) In adipocytes, SIRT1 deacetylates nuclear factor-κB p65 subunit [NF-κB(p65)], resulting in reduced expression of inflammatory mediators such as tumor necrosis factor-α (TNF-α) and chemoattractant protein-1 (MCP-1), and decreased polarization to M1 macrophages and infiltration to adipose tissue. SIRT also induces polarization to M2 macrophages through increased expression of interleukin-4 (IL-4) expression via deacetylation of nuclear factor of activated T-cells 1 (NFATc1). (C) In skeletal muscle, SIRT1 increases mitochondrial biogenesis and fatty acid oxidation through acetylation and activation of the peroxisome proliferator-activated receptor (PPAR)-γ coactivator-1α (PGC-1α). Under conditions of insulin resistance, diabetes, obesity, or aging, mitochondrial oxidative capacity is decreased, contributing to the generation of reactive oxygen species (ROS) in mitochondria. Hyperglycemia, free fatty acids (FFAs) and TNF-α stimulate ROS production from the mitochondria, and increased levels of ROS lead to the serine-phosphorylation of insulin receptor substrate-1 (IRS-1), resulting in reduced insulin signaling. However, SIRT1 interacts with phosphoinositide 3-kinase (PI3K), leading to activation of insulin signaling. Additionally, SIRT1 activates PGC-1α transcriptional activity to induce mitochondrial biogenesis and the induction of antioxidative enzymes, which can inhibit the generation of ROS by mitochondria. Expression of glucose transporter 4 (GLUT4) is enhanced through deacetylation of PGC-1α by SIRT1. Moreover, SIRT1 activates peroxisome proliferator-activated receptor-α (PPAR-α), which induces fatty acid oxidation. (D) SIRT1 deacetylates Forkhead box protein O1 (FOXO1) and enhances its interaction with CCAAT/enhancer binding protein α (C/EBPα), resulting in the enhanced transcription of adiponectin in adipocytes. In skeletal muscle, adiponectin is involved in the regulation of Ca2+ signaling and PGC-1α expression through calcium/calmodulin-dependent protein kinase kinase (CaMKK) and calcium/calmodulin-dependent protein kinase (CaMK) activation. Adiponectin activates SIRT1 through AMPK activation, thereby deacetylating PGC-1α and resulting in mitochondrial biogenesis, increased fatty acid oxidation, and oxidative phosphorylation.