Abstract
Reversible post-translational modification (PTM) is a powerful and ubiquitous mechanism to regulate protein function. The mechanistic basis of the associated functional regulation by PTMs often involves the recruitment of interaction partners that selectively binds the modified protein. Identifying such functionally important protein-protein interactions that are uniquely triggered by PTMs remains difficult due to several technical challenges. To address this, here we develop technology to site-specifically incorporate two distinct noncanonical amino acids into recombinant proteins: one modeling a PTM of interest and the second harboring a photoaffinity probe. Using lysine-23 acetylation of histone 3 as a model system, we show that such dual-labeled “protein probes” can covalently capture its “reader” protein.
Graphical Abstract
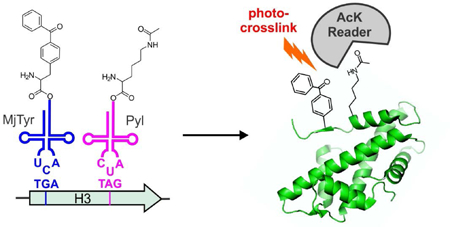
Introduction
Post-translational modifications (PTMs) dramatically expand the chemical space accessible to proteins, and provide a powerful way to regulate protein function in response to specific physiological needs.1–4 The post-translationally modified amino acid residue is often recognized by designated “reader” proteins, and these unique protein-protein interactions (PPIs) orchestrate the functional outcome triggered by specific PTM events (Figure 1a).1–5 Numerous protein domains have evolved to specifically recognize distinct post-translationally modified amino acid residues, such as SH2 domains for phosphotyrosine,6 14-3-3 for phosphoserine,7 bromodomains targeting acetyllysine (AcK),8 etc. Additionally, PTMs can induce dynamic structural changes, and the altered structure can participate in unique PPIs not directly involving the site of modification (Figure 1a).2, 3, 5 Over the last two decades, rapid advances in mass-spectrometry-based proteomics have dramatically expanded both the catalog of unique PTMs known to us, as well as sites in our proteome that are subjected to various PTMs,9–11 but the associated physiological roles remain poorly understood for the most part. Identifying the unique PTM-triggered PPIs remains a particularly challenging problem, because: 1) PTM-induced PPIs are often weak and do not survive pulldown or immunoprecipitation.12, 13 2) Generating a site-specifically PTM-labeled homogeneous protein sample is challenging, as the mechanism by which a newly identified PTM is installed is often unclear or hard to reconstitute. Small synthetic peptides, harboring a PTM of interest in its native sequence context, and further equipped with a photoaffinity probe, have been used with some success to identify PTM-mediated PPIs.12–14 However, such peptide probes would fail to capture PPIs that require the context of the full-length modified protein. The ability to generate analogous full-length “protein probes” incorporating a PTM at a desired sites and further labeled with a photoaffinity probe at a suitable nearby site will provide a powerful approach to systematically explore PTM-triggered PPIs (Figure 1B). Using lysine acetylation as a model PTM, here we describe a strategy to achieve this using the genetic code expansion technology.
Figure 1.
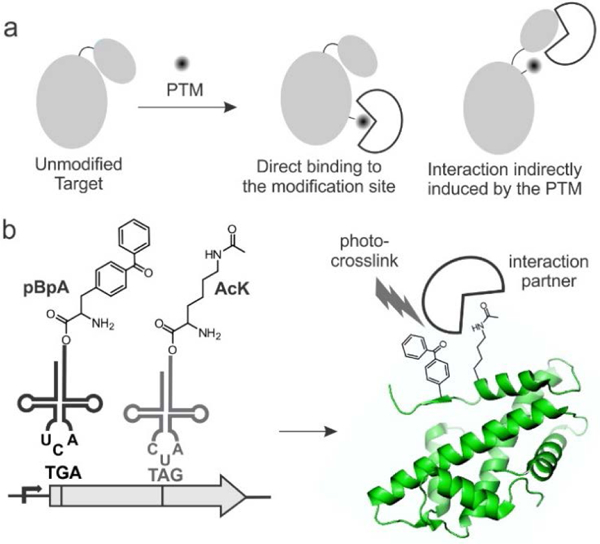
A general strategy to capture PTM-triggered PPIs. a) PTM of proteins can directly or indirectly trigger new PPIs. b) Site-specific incorporation of both a PTM (such as AcK) and a photoaffinity probe (such as pBpA) will yield “protein probes” capable of covalently capturing such PTM-mediated PPIs.
The genetic code expansion technology enables site-specific incorporation noncanonical amino acids (ncAAs) into proteins in living cells using engineered aminoacyl-tRNA synthetase (aaRS)/tRNA pairs that suppress nonsense or frameshift codons.15–17 Using this technology, a variety of PTMs and their structural analogs have been genetically encoded, including Nε-acetyllysine (AcK),18 Nε-crotonyllysine,19 sulfotyrosine,20 phosphotyrosine,21, 22 phosphoserine,23, 24 etc. The ability to co-translationally incorporate these ncAAs into proteins in living cells has created exciting opportunities to probe their physiological consequences with unprecedented precision. Additionally, several photoaffinity probes have also been genetically encoded using this technology, which has been valuable for characterizing PPIs in complex biological systems.15–17, 25 We envisioned that by combining these two powerful abilities, by site-specifically incorporating both a genetically encoded PTM and a photoaffinity probe, it should be possible to create full-length protein probes for covalently capturing PTM-triggered PPIs. To demonstrate the feasibility of this approach, we selected lysine acetylation as the model PTM, since many functionally important lysine acetylations have been characterized in the human proteome.
Site-specific incorporation of two distinct ncAAs into a protein would require the use of two mutually orthogonal aaRS/tRNA pairs, suppressing two different nonsense or frameshift codons. The feasibility of incorporating two distinct ncAAs into proteins in bacteria26–31 as well as mammalian cells32–34 has been demonstrated. In E. coli, the M. jannaschii-derived tyrosyl-tRNA synthetase (MjTyrRS)/tRNA and the Methanosarcina-derived pyrrolysyl-tRNA synthetase (PylRS)/tRNA pair have been used together for dual ncAA incorporation.27–31 An optimized modular three-plasmid system was recently developed for expressing these two pairs (MjTyr and Pyl pairs suppressing TAG and TAA, respectively) and the target protein, which produced double ncAA-modified protein with up to 30% efficiency relative to its wild-type counterpart.27 We reasoned that this platform could be adapted for site-specific co-incorporation of AcK (charged by the Pyl pair) and a photoaffinity probe (e.g., p-benzoylphenylalanine, pBpA, charged by the MjTyr pair).
Results and Discussion
So far, double ncAAs incorporation have been largely limited to proof-of-concept experiments, where well-behaved reporters are expressed with two distinct bioconjugation handles and subsequently functionalized with two distinct entities, such as a FRET pair.27–34 To overcome the intrinsic low efficiency of dual nonsense suppression, such demonstrations have taken advantage of ncAAs that are charged with high efficiency, as well as well-behaved reporter proteins with strategically chosen incorporation sites. Such flexibilities are not available for our goal, since the selection of the target protein, the identity of the ncAAs, and the sites of their incorporation are defined by native PTMs observed in the proteome. Furthermore, the poor activity of the AcK-selective engineered PylRS/tRNA pair (Figure S1) poses another daunting challenge.35 Indeed, when we attempted the expression of a GFP-3TAA-151TAG reporter in E. coli Top10 strain incorporating AcK and pBpA in response to TAA and TAG, respectively, using the aforementioned optimized three-plasmid system, little full-length reporter expression was observed (Figure S2). To overcome the poor AcK incorporation efficiency, we took a two-pronged approach. The wild-type tRNAPyl variant in the pUltra-Pyl suppression plasmid was replaced with a counterpart that has been shown to enhance TAG suppression efficiency in E. coli (Figure 2a).36 Additionally, we took advantage of an E. coli strain devoid of a functional release factor 1 (RF1), which provides improved TAG-suppression levels.37–39 Together, these led to a significant improvement in the incorporation efficiency of AcK, as measured by the the expression of a sfGFP-151-TAG reporter (Figure 2b).
Figure 2.
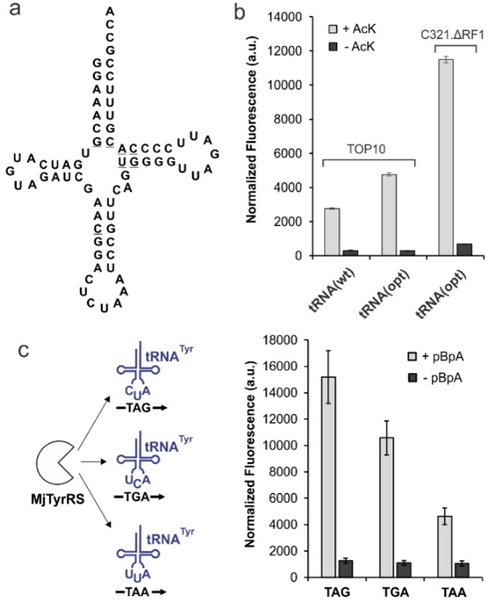
Optimization of an expression system for co-incorporation of AcK and pBpA into proteins in E. coli. a) Structure of an optimized tRNAPyl, with mutations underscored. b) The use of the optimized tRNAPyl and the C321.ΔRF1 strain improves AcK incorporation efficiency, measured by the expression of the full-length sfGFP-151-TAG reporter in the presence of the AcK-selective Pyl-pair (expressed from pUltra). c) The MjTyr pair can be used as an efficient TGA suppressor. The efficiency of suppressing the three different nonsense codons using this pair was evaluated using the sfGFP-151-nonsense mutant expression assay.
Even though both MjTyr and Pyl pairs were developed as TAG suppressors, one of these must suppress a non-TAG codon for incorporating two different ncAAs. So far, the Pyl pair has been exclusively assigned to the non-TAG codon (e.g., TAA or AGGA) in dual ncAA incorporation experiments, since it exhibits broad anticodon-tolerance.27–31 However, to take advantage of the improved AcK incorporation system described above, which relies on the enhanced TAG suppression efficiency in the RF1-deleted E. coli strain, it is imperative to use it as a TAG suppressor. Consequently, we explored if the MjTyr pair can be instead used for suppressing a non-TAG nonsense codon. We constructed TGA and TAA suppressor plasmids (pEVOL) encoding the pBpA-selective MjTyr pair40 and tested pBpA incorporation efficiency using appropriate mutant sfGFP reporters (Figure 2c). The MjTyr pair was found to suppress TGA efficiently, supporting the feasibility of its simultaneous use with the TAG-suppressing Pyl pair.
To evaluate if the Pyl and MjTyr pairs can be used to concurrently incorporate AcK and pBpA in response to TAG and TGA, we co-expressed these with a sfGFP-3TGA-49TAG reporter using a modular three plasmid system: the optimized AcK-selective Pyl pair was expressed from pUltra,27 the pBpA-selective MjTyr pair from pEVOL,41 and the sfGFP reporter from pET22b. We observed robust expression of the full-length reporter protein only when both ncAAs were supplemented in the medium (Figure 3). The full-length reporter was purified by immobilized metal ion-affinity chromatography (IMAC) using a C-terminal polyhistidine tag with excellent yield (90 mg/L), and its MS-analysis was consistent with the incorporation of AcK and pBpA at desired sites (Figure S3).
Figure 3.
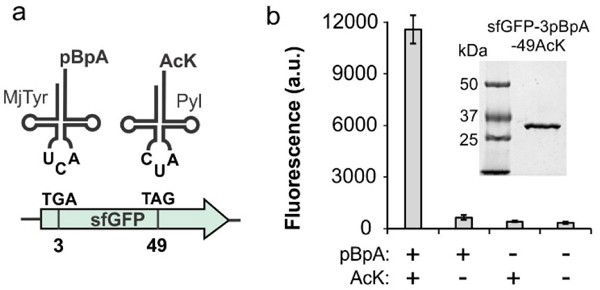
Incorporation of AcK and pBpA into a sfGFP reporter. a) Scheme of dual nAA incorporation. b) Efficient expression of the sfGFP-3TGA-49TAG reporter in RF1-deleted E. coli using optimized pUltra-Pyl(TAG) charging AcK, and pEVOL-MjTyr (TGA) charging pBpA, selectively in the presence of both ncAAs. SDS-PAGE analysis of the purified reporter is also shown.
Having established an efficient expression system to co-incorporate AcK and pBpA into target proteins, we wanted to demonstrate that such dual-labeled proteins could be indeed useful to capture acetylation-triggered PPIs. Lysine acetylation was first discovered in the context of histones,42 and many functionally important histone acetylations has been characterized to date.43–46 For example, acetylation of lysine 23 in human histone 3 (H3) is selectively recognized by the bromodomain in the reader protein tripartite motif-containing 24 (TRIM24; Figure 4a).47 The multifunctional protein TRIM24 is a p53-targeting E3-ubiquitin ligase, and is broadly associated with chromatin silencing. It recognizes both methylation of lysine 4 and acetylation of lysine 23 in H3, using a PHD and a bromodomain, respectively, and activate estrogen-dependent genes important in cell proliferation and tumorigenesis.47 The acetylation-dependent interaction between TRIM24 to H3 provides a great model system to evaluate the feasibility of our strategy.
Figure 4.
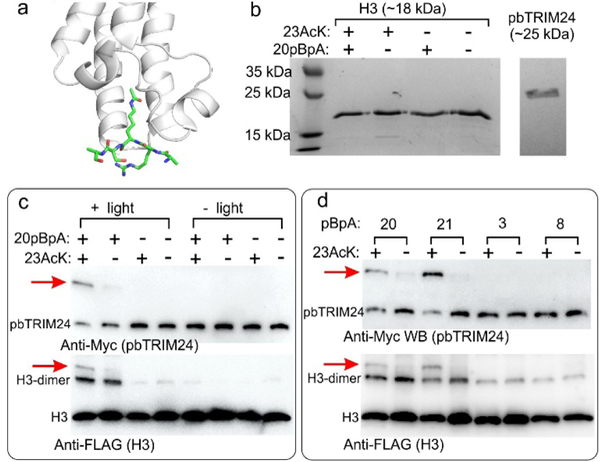
Capturing acetylation-dependent association between pbTRIM24 and H3 using AcK/pBpA-dual labeled H3 probes. a) Crystal structure of pbTRIM24 bound to a H3-derived peptide acetylated at 23. (PDB: 3O34) b) SDS-PAGE analysis of H3 mutants and pbTRIM24. c) H3–20pBpA-23AcK photo-crosslinks with pbTRIM24 in an acetylation-dependent manner, as detected by western blot (the appropriately sized crosslinked product is highlighted by arrow). H3 and pbTRIM24 are selectively detected using C-terminal FLAG and Myc tags, respectively. H3 was used in excess to compensate for its aggregation-prone nature. d) Crosslinking efficiency between H3–23AcK and pbTRIM24 is sensitive to the site of pBpA incorporation: Efficient acetylation-dependent crosslink formation is observed when pBpA is incorporated at 20 and 21, but not at 3 and 8. Magnified pictures of these gels are shown in Figure S7.
To express H3 incorporating AcK and pBpA, we first replaced the sfGFP reporter in our expression system with wild-type human H3 fused at the C-terminus with a polyhistidine-tag and the N-terminus with a FLAG tag, and confirmed our ability to express and purify this protein with good yield (65 mg/L; Figure S4, Table S1). Next, four double nonsense mutants of H3 were created – each with TAG at position 23 to incorporate AcK, but TGA at positions 20, 21, 3 or 8 (to incorporate pBpA). Sites 20 and 21 are proximal to the acetylation site and could enable crosslinking to the bromodomain, while 3 and 8 are on a region that associates with the PHD domain (Figure S6). Using our optimized dual-ncAA incorporation system, we were able to achieve robust expression of all four double nonsense mutants of H3 (11–30 mg/L; Figure 4b, Table S1). In each case, the mutant protein was purified and characterized by MS analysis to verify desired ncAA incorporation (Figure S4).
Next, to explore the feasibility of capturing the AcK-dependent interaction between H3 and TRIM24, we first cloned, expressed and purified the PHD-bromodomain of TRIM24 (pbTRIM24) with a C-terminal tandem Myc-polyhistidine tag (Figure 4b, Figure S5).47 In addition to the double nonsense mutants of the H3 proteins described above, we also expressed the corresponding single mutants (incorporating AcK or pBpA alone; Figure 4b, Figure S4, Table S1) as controls. When pbTRIM24 was incubated with various H3 mutants and irradiated (365 nm), formation of the appropriate crosslinked product (between H3 and pbTRIM24) was observed by western blot (detecting Flag-H3 or Myc-pbTRIM24) only for H3 double mutant incorporating AcK and pBpA (Figure 4c). The crosslinking efficiency was also found to be sensitive to the position of pBpA, as might be expected: at sites 20 and 21 it produced efficient crosslinking, but not at sites 3 and 8 (Figure 4d). The ability to optimize crosslinking efficiency by systematically altering the position of the photo-crosslinker represents a major strength of this approach. This feature can also be used to map the interacting regions, when structural information is unavailable. These experiments corroborate the feasibility of using PTM/photoaffinity probe double-labeled proteins as “probes” to capture PTM-mediated PPIs.
In summary, we have developed a novel approach to covalently capture PTM-dependent PPIs, by co-translationally incorporating both a PTM (AcK) and a photoaffinity probe into full-length proteins – a first to our knowledge. To this end, we have significantly enhanced the scope of dual ncAA incorporation in E. coli by developing an optimized expression platform. The same platform can also be readily adapted to other genetically encoded lysine-PTMs such as Nε-crotonyllysine19 and Nε−2-hydroxyisobutyryllysine,48 the physiological roles for which remain incompletely understood. Owing to the modular nature of our expression system, it should be also possible to extend it to other genetically encoded PTMs such as phosphotyrosine. We are currently developing strategies to use these dual-labeled “protein probes” to identify unknown PTM-associated PPIs from mammalian cell-free extracts using quantitative mass-spectrometry-based proteomics. Additionally, efforts are under way to extend this technology to mammalian cells, such that dual-labeled protein probes can be expressed directly in its native context. The ability to i) homogeneously install a genetically encoded PTM into the desired site of any full-length protein, and ii) systematically alter the placement of the photo-crosslinker probe relative to the PTM site, to ensure optimal light-induced covalent capture of the binding partner, makes this approach highly attractive to explore the PTM-associated PPIs that underpin the complex regulatory network of our biology.
Supplementary Material
Acknowledgement
We thank NIH (R01GM124319 to A.C.) for financial support. We thank Prof. R. Mehl, Prof. P. Schultz, and Prof. G. Church for kindly providing the pBK-AcKRS3 plasmid, the pEVOL-pBpA plasmid, and the C321.RF1- strain, respectively.
Footnotes
Supporting Information Available. This material is available free of charge via the Internet.
References
- 1.Beltrao P, Bork P, Krogan NJ, and van Noort V (2013) Evolution and functional cross-talk of protein post-translational modifications, Molecular systems biology 9, 714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deribe YL, Pawson T, and Dikic I (2010) Post-translational modifications in signal integration, Nature structural & molecular biology 17, 666–672. [DOI] [PubMed] [Google Scholar]
- 3.Sims RJ 3rd, and Reinberg D (2008) Is there a code embedded in proteins that is based on post-translational modifications?, Nature reviews. Molecular cell biology 9, 815–820. [DOI] [PubMed] [Google Scholar]
- 4.Walsh CT, Garneau-Tsodikova S, and Gatto GJ Jr. (2005) Protein posttranslational modifications: the chemistry of proteome diversifications, Angewandte Chemie (International ed. in English) 44, 7342–7372. [DOI] [PubMed] [Google Scholar]
- 5.Seet BT, Dikic I, Zhou MM, and Pawson T (2006) Reading protein modifications with interaction domains,Nature reviews. Molecular cell biology 7, 473–483. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe MB (2002) Phosphotyrosine-binding domains in signal transduction, Nature reviews. Molecular cell biology 3, 177–186. [DOI] [PubMed] [Google Scholar]
- 7.Yaffe MB, and Smerdon SJ (2001) PhosphoSerine/threonine binding domains: you can’t pSERious?, Structure (London, England : 1993) 9, R33–38. [DOI] [PubMed] [Google Scholar]
- 8.Filippakopoulos P, and Knapp S (2012) The bromodomain interaction module, FEBS letters 586, 2692–2704. [DOI] [PubMed] [Google Scholar]
- 9.Larance M, and Lamond AI (2015) Multidimensional proteomics for cell biology, Nature reviews. Molecular cell biology 16, 269–280. [DOI] [PubMed] [Google Scholar]
- 10.Olsen JV, and Mann M (2013) Status of large-scale analysis of post-translational modifications by mass spectrometry, Molecular & cellular proteomics : MCP 12, 3444–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mertins P, Qiao JW, Patel J, Udeshi ND, Clauser KR, Mani DR, Burgess MW, Gillette MA, Jaffe JD, and Carr SA (2013) Integrated proteomic analysis of post-translational modifications by serial enrichment, Nature methods 10, 634–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Foley EA, Kawashima SA, Molloy KR, Li Y, Chait BT, and Kapoor TM (2013) Examining post-translational modification-mediated protein-protein interactions using a chemical proteomics approach, Protein science : a publication of the Protein Society 22, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Foley EA, Molloy KR, Li Y, Chait BT, and Kapoor TM (2012) Quantitative chemical proteomics approach to identify post-translational modification-mediated protein-protein interactions, Journal of the American Chemical Society 134, 1982–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao X, Wang Y, Li X, Li XM, Liu Z, Yang T, Wong CF, Zhang J, Hao Q, and Li XD (2014) Identification of ‘erasers’ for lysine crotonylated histone marks using a chemical proteomics approach, eLife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chin JW (2017) Expanding and reprogramming the genetic code, Nature 550, 53–60. [DOI] [PubMed] [Google Scholar]
- 16.Dumas A, Lercher L, Spicer CD, and Davis BG (2015) Designing logical codon reassignment–Expanding the chemistry in biology, Chemical Science 6, 50–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young DD, and Schultz PG (2018) Playing with the Molecules of Life, ACS chemical biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann H, Peak-Chew SY, and Chin JW (2008) Genetically encoding N(epsilon)-acetyllysine in recombinant proteins, Nature chemical biology 4, 232–234. [DOI] [PubMed] [Google Scholar]
- 19.Kim CH, Kang M, Kim HJ, Chatterjee A, and Schultz PG (2012) Site-specific incorporation of epsilon-N-crotonyllysine into histones, Angewandte Chemie (International ed. in English) 51, 7246–7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu CC, and Schultz PG (2006) Recombinant expression of selectively sulfated proteins in Escherichia coli, Nature biotechnology 24, 1436–1440. [DOI] [PubMed] [Google Scholar]
- 21.Luo X, Fu G, Wang RE, Zhu X, Zambaldo C, Liu R, Liu T, Lyu X, Du J, and Xuan W (2017) Genetically encoding phosphotyrosine and its nonhydrolyzable analog in bacteria, Nature chemical biology 13, 845–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoppmann C, Wong A, Yang B, Li S, Hunter T, Shokat KM, and Wang L (2017) Site-specific incorporation of phosphotyrosine using an expanded genetic code, Nature chemical biology 13, 842–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park HS, Hohn MJ, Umehara T, Guo LT, Osborne EM, Benner J, Noren CJ, Rinehart J, and Soll D (2011) Expanding the genetic code of Escherichia coli with phosphoserine, Science (New York, N.Y.) 333, 1151–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogerson DT, Sachdeva A, Wang K, Haq T, Kazlauskaite A, Hancock SM, Huguenin-Dezot N, Muqit MM, Fry AM, Bayliss R, and Chin JW (2015) Efficient genetic encoding of phosphoserine and its nonhydrolyzable analog, Nature chemical biology 11, 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, Song H, and Chen PR (2016) Genetically encoded photocrosslinkers for identifying and mapping protein-protein interactions in living cells, IUBMB life 68, 879–886. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JC, Wu N, Santoro SW, Lakshman V, King DS, and Schultz PG (2004) An expanded genetic code with a functional quadruplet codon, Proceedings of the National Academy of Sciences of the United States of America 101, 7566–7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee A, Sun SB, Furman JL, Xiao H, and Schultz PG (2013) A versatile platform for single- and multiple-unnatural amino acid mutagenesis in Escherichia coli, Biochemistry 52, 1828–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumann H, Wang K, Davis L, Garcia-Alai M, and Chin JW (2010) Encoding multiple unnatural amino acids via evolution of a quadruplet-decoding ribosome, Nature 464, 441–444. [DOI] [PubMed] [Google Scholar]
- 29.Sachdeva A, Wang K, Elliott T, and Chin JW (2014) Concerted, rapid, quantitative, and site-specific dual labeling of proteins, Journal of the American Chemical Society 136, 7785–7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan W, Huang Y, Wang Z, Russell WK, Pai PJ, Russell DH, and Liu WR (2010) A facile system for genetic incorporation of two different noncanonical amino acids into one protein in Escherichia coli, Angewandte Chemie (International ed. in English) 49, 3211–3214. [DOI] [PubMed] [Google Scholar]
- 31.Wang K, Sachdeva A, Cox DJ, Wilf NM, Lang K, Wallace S, Mehl RA, and Chin JW (2014) Optimized orthogonal translation of unnatural amino acids enables spontaneous protein double-labelling and FRET, Nature chemistry 6, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao H, Chatterjee A, Choi SH, Bajjuri KM, Sinha SC, and Schultz PG (2013) Genetic incorporation of multiple unnatural amino acids into proteins in mammalian cells, Angewandte Chemie (International ed. in English) 52, 14080–14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng Y, Addy PS, Mukherjee R, and Chatterjee A (2017) Defining the current scope and limitations of dual noncanonical amino acid mutagenesis in mammalian cells, Chem Sci 8, 7211–7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Y, Mukherjee R, Chin MA, Igo P, Gilgenast MJ, and Chatterjee A (2018) Expanding the scope of single and dual noncanonical amino acid mutagenesis in mammalian cells using orthogonal polyspecific leucyl-tRNA synthetases, Biochemistry 57, 441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umehara T, Kim J, Lee S, Guo LT, Soll D, and Park HS (2012) N-acetyl lysyl-tRNA synthetases evolved by a CcdB-based selection possess N-acetyl lysine specificity in vitro and in vivo, FEBS letters 586, 729–733. [DOI] [PubMed] [Google Scholar]
- 36.Fan C, Xiong H, Reynolds NM, and Soll D (2015) Rationally evolving tRNAPyl for efficient incorporation of noncanonical amino acids, Nucleic acids research 43, e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lajoie MJ, Rovner AJ, Goodman DB, Aerni HR, Haimovich AD, Kuznetsov G, Mercer JA, Wang HH, Carr PA, Mosberg JA, Rohland N, Schultz PG, Jacobson JM, Rinehart J, Church GM, and Isaacs FJ (2013) Genomically recoded organisms expand biological functions, Science (New York, N.Y.) 342, 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng Y, Lajoie MJ, Italia JS, Chin MA, Church GM, and Chatterjee A (2016) Performance of optimized noncanonical amino acid mutagenesis systems in the absence of release factor 1, Molecular bioSystems 12, 1746–1749. [DOI] [PubMed] [Google Scholar]
- 39.Amiram M, Haimovich AD, Fan C, Wang Y-S, Aerni H-R, Ntai I, Moonan DW, Ma NJ, Rovner AJ, and Hong SH (2015) Evolution of translation machinery in recoded bacteria enables multi-site incorporation of nonstandard amino acids, Nature biotechnology 33, 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chin JW, Martin AB, King DS, Wang L, and Schultz PG (2002) Addition of a photocrosslinking amino acid to the genetic code of Escherichiacoli, Proceedings of the National Academy of Sciences of the United States of America 99, 11020–11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young TS, Ahmad I, Yin JA, and Schultz PG (2010) An enhanced system for unnatural amino acid mutagenesis in E. coli, Journal of molecular biology 395, 361–374. [DOI] [PubMed] [Google Scholar]
- 42.Allfrey V, Faulkner R, and Mirsky A (1964) Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis, Proceedings of the National Academy of Sciences 51, 786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bannister AJ, and Kouzarides T (2011) Regulation of chromatin by histone modifications, Cell research 21, 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Filippakopoulos P, Picaud S, Mangos M, Keates T, Lambert JP, Barsyte-Lovejoy D, Felletar I, Volkmer R, Muller S, Pawson T, Gingras AC, Arrowsmith CH, and Knapp S (2012) Histone recognition and large-scale structural analysis of the human bromodomain family, Cell 149, 214–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strahl BD, and Allis CD (2000) The language of covalent histone modifications, Nature 403, 41–45. [DOI] [PubMed] [Google Scholar]
- 46.Yang XJ, and Seto E (2008) Lysine acetylation: codified crosstalk with other posttranslational modifications, Molecular cell 31, 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai WW, Wang Z, Yiu TT, Akdemir KC, Xia W, Winter S, Tsai CY, Shi X, Schwarzer D, Plunkett W, Aronow B, Gozani O, Fischle W, Hung MC, Patel DJ, and Barton MC (2010) TRIM24 links a non-canonical histone signature to breast cancer, Nature 468, 927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao H, Xuan W, Shao S, Liu T, and Schultz PG (2015) Genetic Incorporation of epsilon-N-2-Hydroxyisobutyryl-lysine into Recombinant Histones, ACS chemical biology 10, 1599–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


