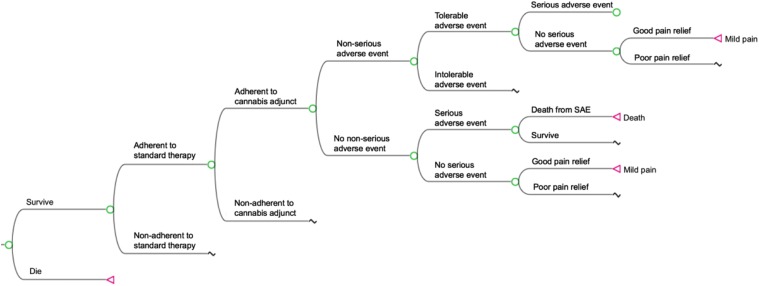
FIG. 1.
Abbreviated model overview. Pictured is a node structure wherein adjunctive cannabis is integrated into a treatment model using standard therapy agents described by Bellows et al.31 Beginning in a moderate-to-severe pain health state, simulated patients are assessed stepwise for mortality, adherence, tolerable or intolerable adverse events, SAE, and quality of pain relief. Patients who die are removed from the simulation and do not transition further. Nonadherence disqualifies a patient from experiencing either pain relief or adverse events from a given agent. Serious or intolerable adverse events trigger discontinuation of current therapy (with or without adjunctive cannabis) and drug-switching. Patients who attain good pain relief (pain score <4) transition to the mild pain state at the end of the cycle. In the absence of good pain relief, patients remain in moderate-to-severe pain at the end of the cycle. SAE, serious adverse events.