Figure EV2. The cytoplasmic inactive form of Rab8a is degraded by the proteasome pathway (related to Figs 2, 3, 4).
-
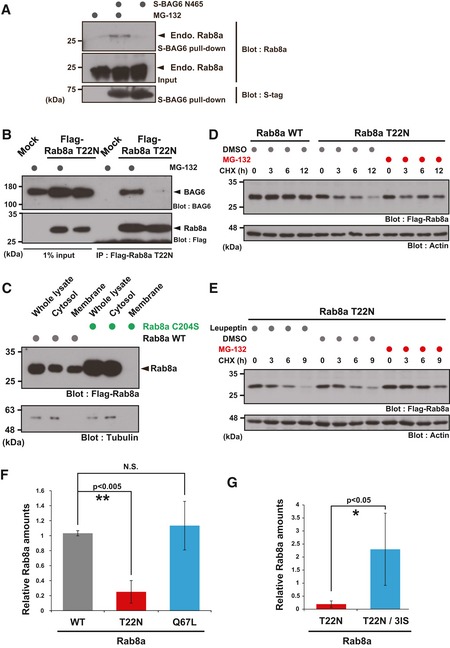
AEndogenous Rab8a protein was co‐precipitated with S‐tagged BAG6 N465 from HeLa cell lysate in the presence of MG‐132.
-
BCo‐immunoprecipitation of BAG6 with Rab8a was stimulated by the addition of a proteasome inhibitor. HeLa cells expressing Flag‐tagged Rab8a (T22N) protein were treated with (+) or without (−) 10 μM MG‐132. At 4 h after MG‐132 treatment, the cells were lysed and Flag‐precipitates were probed with an anti‐BAG6 antibody.
-
CRab8a C204S mutant protein localized exclusively in the soluble cytoplasmic fraction. Whole cell extracts of HeLa cells expressing Flag‐tagged Rab8a proteins (WT or C204S mutant) were fractionated into the cytosolic and membrane fractions. Tubulin was used as a cytoplasmic marker.
-
D, ET22N mutant form of full‐length Rab8a protein was stabilized by MG‐132, while leupeptin did not affect its stability. At 24 h after Rab8a transfection, the cells were cultured with 10 μM MG‐132, 10 μM leupeptin, or an equivalent amount of DMSO (as a negative control), and then chased with 20 μg/ml CHX and harvested at the indicated times after CHX addition.
- F, G
Source data are available online for this figure.
