Figure 5. Endogenous BAG6 is necessary for the elimination of cytosolic Rab8a.
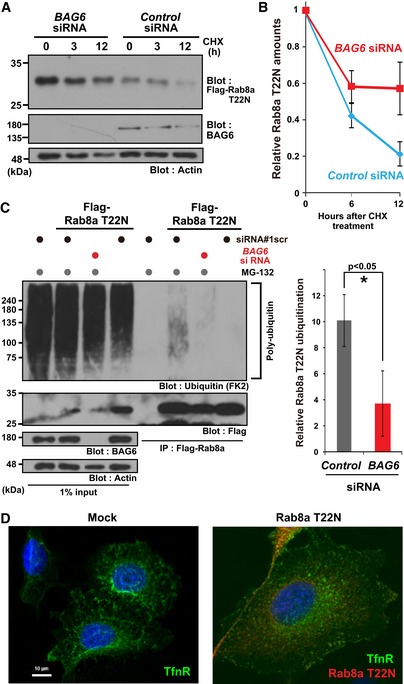
- Rab8a (T22N) protein accumulated in BAG6‐knockdown cells. HeLa cells were transfected with siRNA duplexes for BAG6 or control siRNA. At 48 h after siRNA transfection, Flag‐tagged‐Rab8a (T22N) was expressed in the cells. At 24 h after Rab8a (T22N) transfection, the cells were chased with 50 μg/ml CHX and harvested at the indicated time after CHX addition. Actin was used as a loading control.
- Anti‐Flag blot signals in the control or BAG6 siRNA‐treated cells were quantified, and relative signal intensities after CHX addition were calculated. The value of the Flag‐signal at 0 h was defined as 1.0. Note that all signal intensities of the Flag‐tag were normalized by that of actin, a loading control, in each sample. The graph represents the mean ± SE calculated from six independent biological replicates. These data were analyzed by Welch's t‐test.
- Polyubiquitin modification of Rab8a was abolished in BAG6‐knockdown cells. Flag‐Rab8a (T22N) immunoprecipitates were blotted with an anti‐polyubiquitin antibody (FK2, left panel). As a negative control, siRNA#1scr was used. Anti‐polyubiquitin signals co‐precipitated with Rab8a (T22N) (a representative example is shown in the left panel) were quantified (right panel). Note that the intensities of the co‐precipitated polyubiquitin signal were normalized both by the input ubiquitin‐signal and bait Flag‐signal. The graph represents the mean ± SD calculated from three independent biological replicates. An asterisk indicates P < 0.05 (Student's t‐test).
- Defective distribution of the endosomal protein TfnR in HeLa cells with the excess accumulation of the inactive form of Rab8a. Right panel shows a merged image of TfnR staining (shown as green), Rab8a (T22N) staining (magenta), and Hoechst 33342 nuclear staining (blue). Scale bar: 10 μm.
Source data are available online for this figure.
