Fig. 1.
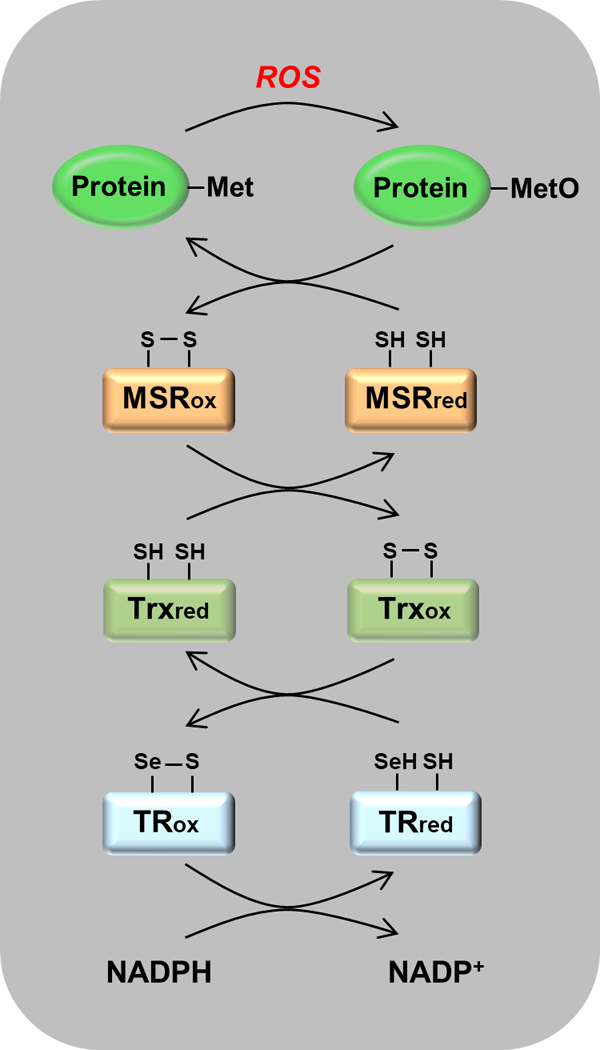
Scavenging of reactive oxygen species (ROS) by the msr-dependent catalytic cascade. Reduced forms of the proteins carry the subscript “red” and oxidized forms carry “ox”. Reading from top to bottom, an ROS is intercepted by a Met residue that is oxidized to MetO. MetO is reduced back to Met by msr, with the formation of a disulfide bond. The oxidized msr is reduced by thioredoxin (Trx), which now carries the disulfide bond. It is reduced by thioredoxin reductase (TR), which in mammals contains a selenocysteine residue that is oxidized, forming a selenocysteine-cysteine bond. This disulfide analogue is then reduced by NADPH. The net result is that ROS is reduced at the expense of NADPH.
