Abstract
Optimal outcomes of a cataract surgery largely depend on the successful performance of an anterior capsulotomy. It is one of the most important steps of modern cataract surgery which reduces the risk of capsular tears and ensures postoperative stable intraocular lens (IOL). Anterior capsulotomy is considered ideal if it is round, continuous, well-centered, and overlaps the implanted IOL around its circumference. If any of these features is missing, it can be a cause of impedance for desired surgical and visual outcomes. Manual can opener and manual capsulorhexis are the routine standard techniques employed for manual extracapsular cataract extraction and phacoemulsification, respectively. Recent increasing use of femtosecond laser cataract surgery has allowed cataract surgeons to obviate inherent inaccuracies of manual anterior capsulotomy techniques. There is an ongoing quest to find an ideal, risk free, and surgeon-friendly technique of anterior capsulotomy that can be employed for surgery in all types of cataracts.
Keywords: Capsulorhexis, capsulotomy, Femto laser, pediatric capsulotomy, plasma blade, Zepto laser
Cataract surgery is the most commonly performed ophthalmic procedure worldwide with major anatomical and optical goal being, a well-centered posterior chamber intraocular lens (IOL). Anterior capsulotomy has evolved from an era of can-opener capsulotomy to circular capsulorhexis (CCC) and more recently to precision pulse anterior capsulotomy. A smooth-edged capsulotomy renders the capsular bag opening its strength, for subsequent steps of phacoemulsification and is considered ideal if it is round, continuous, well-centered, and overlaps the IOL around its circumference.
In this review, we describe various techniques of anterior capsulotomy along with variations in special circumstances --- pediatric cataracts, white cataracts, and cataracts with small pupils.
Types and Modalities of Anterior Capsulotomy in Cataract Surgery
Vogt described the earliest technique of capsulotomy by utilizing toothed forceps for tearing out a part of anterior capsule. However, this technique was documented to cause occurrence of significant capsular complications.[1] Subsequently, Kelman in 1968 reported “Christmas tree” approach, in which a cystitome was used to peel anterior capsule to create a triangular opening in a Christmas tree morphology.[2] Further innovations targeted to improve on this technique by creating a smooth, circular, and central anterior capsulotomy.
Can opener capsulotomy
Jacques Daviel in 1752 described can opener capsulotomy as a circular ragged opening fashioned by using a cystitome. This technique is commonly employed for doing extracapsular cataract surgery.
Technique
The technique involves creation of a circular opening 5–6 mm in diameter by series of small tears in the anterior capsule with a cystitome [Fig. 1]. Cystitome can be made by either bending a 26-gauge needle or using a prefabricated commercially available cystitome.
Figure 1.
Can opener capsulotomy is performed by making a series of small tears in the anterior capsule using a cystitome
Morphological features
As indicated by its name the can opener capsulotomy is a circular ragged opening with multiple irregular freely mobile capsular tags. These ragged edges of the torn capsule are a source of inherent weakness that can predispose to occurrence of radial tears if there is an increase in intracapsular pressure.
Complications
While can opener capsulotomy is easy to perform, the characteristic weakness associated with its ragged edges makes it unsuitable for phacoemulsification. The main complication associated with can opener capsulotomy is occurrence of anterior capsular radial tears. These tears make the capsular bag unstable and besides interfering in aspiration of cortical matter can also affect the centration of IOL. Moreover, if significant pressure is created in capsular bag, these radial tears can extend through zonules causing occurrence of catastrophic posterior capsular (PC) tears. Also, tags can occlude aspiration port and impede removal of cortical lens matter. If too much aspiration force is used during cortical clean-up, anterior capsular tags can be pulled, and cause PC ruptures, vitreous disturbance and pea podding of IOL.
Envelope capsulotomy
The concept of envelope capsulotomy was suggested by Sourdilla and Baikuff in 1979. However, Galand[3] developed it to its present stage and popularized the technique. It's a safer version of can opener capsulotomy that reduces the risk of capsular zip and corneal endothelial damage by facilitating intercapsular aspiration of cortical matter.
Technique
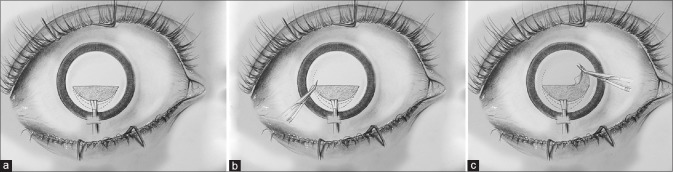
Envelope capsulotomy involves making a linear incision in upper one-third of anterior capsule, followed by nucleus delivery and intercapsular aspiration of cortical matter. Unlike can opener capsulotomy, it can ensure a successful in the bag implantation of a posterior chamber IOL. After complete removal of cataract, radial cuts are made at ends of incision with Vannas scissors and capsular flap is torn off like in capsulorhexis [Fig. 2a–c]. Major advantages include minimal trauma to corneal endothelium and a good centration of IOL.[4] Envelope technique is considered to be very useful in white cataracts where it provides a scaffold for nucleus removal and lens implantation.[5]
Figure 2.
(a–c) Envelope capsulotomy involves making a linear incision in the upper one-third of the anterior capsule. After removal of the nucleus and cortical matter, radial cuts are made and the capsular flap is torn similar to capsulorhexis
Morphological features
As its name suggests, this capsulotomy has an envelope like architecture that allows intercapsular delivery of nucleus and in the bag implantation of IOL It is also known as antismile capsulotomy and unlike can opener capsulotomy occurrence of radial anterior capsular tears with this technique is a rare phenomena.
Complications
An inadvertent occurrence of asymmetrical capsular flaps may cause upward decentration of IOL. Sometimes, incompletely removed capsular flaps can generate free floating capsular tags that get stuck to the pupillary margin causing postoperative dyscoria.[5] Studies comparing the effect of two different anterior capsulotomy techniques, i.e. CCC and envelope on IOL tilt and decentration reported that envelope technique was associated with more incidence of IOL tilt and decentration.[6] Incidences of zonular disruption have also been reported to occur more commonly in envelope capsulotomy as compared to CCC.[7,8]
Manual capsulorhexis
Development of continuous CCC technique has significantly contributed to the safety and effectiveness of modern cataract extraction by phacoemulsification. Since it was first described by Gimbel and Neuhann, CCC has become the technique of choice for anterior capsulotomy.[9] Capsulorhexis as described by Gimbel and Neuhann, is a circular, central, curvilinear opening in the anterior capsule created with a subincisional needle puncture and then completed with arcuate shearing taken in clockwise and anticlockwise directions.[10] A well-centered, adequately sized continuous capsulorhexis is a prerequisite for successful phacoemulsification. It ensures a safe and effective performance of various steps of surgery with a correctly positioned IOL that has optimal rotational stability.[9,10,11] CCC can efficiently create different sizes of smooth and circular capsulotomy with a strong capsular rim that relatively resists tearing even when stretched during cortical removal and lens implantation.
Technique
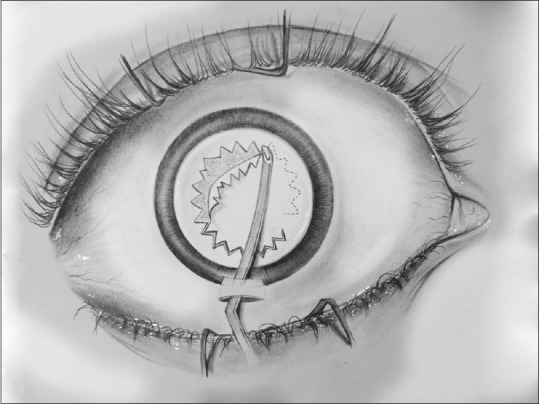
Manually, the capsulorhexis can be fashioned by creating a small tear in the center of the anterior capsule and later advancing the resulting capsular flap into a circular shape by guiding the leading edge with the cystitome [Fig. 3]. Alternatively, the capsular flap can be pulled in a circular fashion by grasping the leading edge with a forceps and advancing the tear with frequent regrasping. Various instruments and devices have been developed to facilitate the creation of a CCC that include needle capsulorhexis,[12] forceps capsulorhexis,[13] bimanual capsulorhexis,[14] and two-staged capsulorhexis.[15] Several mechanized systems have also been developed to facilitate CCC.[16]
Figure 3.
Continuous curvilinear capsulorhexis can be fashioned by creating a small tear in the center of the anterior capsule and ladvancing the resulting capsular flap into a circular shape by guiding the leading edge with the cystitome or by by grasping the leading edge with a forceps and advancing the tear with frequent regrasping
Morphological features
As compared to other techniques of capsulotomy, CCC is considered to be stronger because of continuous smoothness of its edges [Fig. 4]. The architecture of capsulotomy greatly influences the position of lens and subsequent refractive outcome.[17] A perfectly circular and properly sized capsulorhexis allows the capsular bag to completely envelop the optic providing a more predictable effective lens position (ELP) and achieving optimal refractive outcome [Table 1]. In addition, it also reduces the occurrence of posterior capsular opacification (PCO).[18,19,20]
Figure 4.
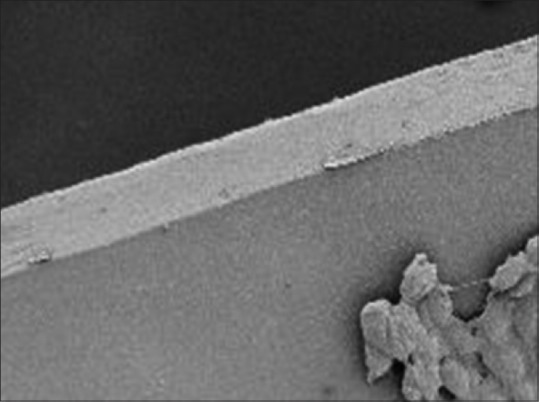
Scanning electron microscopy of manual capsulorhexis demonstrating the smoothness of the edges
Table 1.
Overview of various studies highlighting complications encountered in anterior capsulotomy techniques
| Type of capsulotomy | Author | Study type | Sample size | Complications encountered |
|---|---|---|---|---|
| Envelope | Ndiaye et al. 1999[5] | Prospective | 25 eyes | Postoperative dyscoria |
| Envelope | Akkin et al. 1994[6] | Prospective | 65 eyes | IOL tilt and decentration |
| Comparative evaluation of manual capsulotomies | Oner et al. 2001[7] | Prospective | 95 eyes | Lens decentration more in capsulotomy types other then CCC |
| Manual CCC | Cekic et al. 1999[21] | Prospective | 51 eyes | Altered effective lens position (ELP) in inadequate sized capsulotomies |
| Manual CCC | Wirtitsch et al. 2004[22] | Prospective | 104 eyes | Dysphotopsias and compromised retinal images in decentered capsulotomy |
| Manual CCC | Hollick 1999[18] | Prospective | 75 eyes | Large capsulorhexis associated with Posterior capsule wrinkling and PCO |
| Manual CCC | Olali et al. 2007[27] | Prospective | 358 eyes | Breach rhexis in 0.56% cases |
| Femto scond laser | Chang et al. 2014[41] | Retrospective | 170 eyes | Free-floating capsule buttons in 88.8%. Radial anterior capsule tears in 5.3% |
| Femto scond laser | Abell et al. 2014[43] | Prospective cohort | 1626 eyes | Increased rate of anterior capsule tears |
| Femto second laser | Roberts et al. 2011[50] | Prospective | 50 eyes | Capsular block syndrome |
| Plasma blade | Izak et al. 2004[52] | Experimental | 4 porcine eyes | Capsulotomy lacks elastic stiffness |
| Precision Pulse Capsulotomy | Hooshmand et al. 2018[57] | Prospective, multicenter case series | 158 eyes | Incomplete capsulotomy and radial tear. |
CCC – Central circular capsulorhexis; IOL – Intra ocular lens; PCO – Posterior capsular opacification
If the CCC is too small, IOL may be pushed posteriorly by excessive anterior capsular overlap, thereby causing an alteration in the ELP leading to a hyperopic shift. If it is too large, the unrestrained optic can reposition more anteriorly, resulting in a myopic outcome. If the capsulotomy is decentered, the IOL optic can be tilted or decentred, resulting in astigmatism or compromised retinal image.[21,22] In addition, this could lead to occurrence of negative dysphotopsias. Henderson et al. found the optimal lens position to be centered in the bag with one optic–haptic junction at an inferotemporal position.[23] The relationship between capsulorhexis and refractive outcome is based on inverse sizing of the capsulorhexis. Okada et al.[24] concluded that the spherocylindrical refractive outcome was not related to centration/circularity. They did find however, that decentration of optic center by 0.4 mm was associated with 0.25D change in spherical equivalent.
The appropriate size of capsulotomy compatible with phacoemulsification procedures varies between 5 and 6 mm, with an ideal size of 5.5 mm. In eyes with weak zonules, the capsulorhexis can be kept 0.5–1.0 mm larger than the optic to prevent capsule contracture.[12] Small capsulorhexis (4.5–5.0 mm) has been reported to be associated with less wrinkling and less PCO when compared to large capsulorhexis.[18,19] In cataract surgery with polymethylmethacrylate IOL implantation, a small capsulorhexis with complete capsule/optic overlap is preferable in reducing PCO.[19]
However, a small capsulotomy has multiple intraoperative and postoperative disadvantages. Intraoperatively, it makes surgery more difficult with a smaller opening for nuclear disassembly maneuvers and also increase stress or stretching forces on the anterior capsule edge.[20] Postoperatively, a small capsulorhexis is more likely to contract rapidly and cause occurrence of capsular contraction syndrome.[25] Size of capsulorhexis remains an important determinant of strength, integrity, and transparency of capsule. Tan et al.[26] found faster lens fiber regeneration in smaller anterior capsulotomy diameter in an animal model. Although a 5.5-mm capsulorhexis may be the perfect size of the capsulotomy, different conditions may warrant different sizes. For dense cataracts, a larger capsulorhexis is preferable for safer intraoperative outcomes as opposed to slightly smaller capsulorhexis in posterior polar cataracts to aim for potential placement of IOL in sulcus.[26]
Complications
Like in can opener capsulotomy, anterior capsular radial tears can occur with CCC, if the CCC margin is stretched too much and the reported rates of anterior capsular tears range from 0.5% to 5.6%.[27] Marques et al.[28] found a 48% association with extension of anterior capsular tears to the PC and of these about 19% of patients required an anterior vitrectomy.
Capsulorhexis tears can occur during any stage of surgery and Marques et al.[28] observed capsulorhexis as the most frequent step during which a radial tear occurs. It is well established that radial tears increase the rate of intraoperative and postoperative complications.[29] Many tears can extend to the equator and beyond due to mechanical forces, irrigating fluid, and the intercapsular pressure created by lens material and its manipulation. Early recognition and appropriate management of these tears prevents extension to the PC and subsequent compromise of the structural integrity of the capsular bag.[29,30]
In the event of occurrence of peripheral extension of tear, capsulorhexis forceps can be used to change the direction of tear by holding the torn flap as near to the base as possible and pulling it toward the center of the pupil or by making a midway tangential anterior capsular flap and connecting it to the initial flap.[29,31] Another widely preferred technique is Little's rescue technique,[32] where the capsulorhexis is unfolded into its natural position, and then grasped and pulled backward circumferentially and then inward toward the center. Other techniques include; cystitome reversal technique,[33] reversing the force vector on the capsule flap using a microforceps in a counter clock-wise direction[28] and using Trypan blue to find the leading edge of a lost capsule.[34] In many cases, the radial tear may still extend to the peripheral part of lens mostly due to an increase in intracapsular pressure.[35,36]
Femto second laser-assisted capsulotomy
With new technology comes a drive for more consistent outcomes than that are achievable by an experienced surgeon, and the possibility of a perfectly sized and perfectly circular anterior capsulotomy [Fig. 5]. Capsulotomies created by femtosecond laser are considered to have less variation in centration and size, with reproducible, uniformly circular, and precise diameter when compared to manual CCC.[37] This should theoretically reduce the risk of radial tears, improve capsule overlap and therefore reduce PCO rate, and improve refractive and visual outcomes due to more predictable ELP. Achieved capsulotomy diameters have been shown to be very close to the intended measurements.[38]
Figure 5.
Femtosecond laser capsulotomy has an inherent architecture of near-continuous series of postage-stamp like microperforations
Technique
Currently, there are four commercial systems available for femtosecond capsulorhexis- using optical coherence tomography and Scheimpflug cameras for viewing the capsule anatomy during the procedure. The later provides better view for the posterior capsule at the same time.[39,40] However, the high cost of equipment can make them less affordable for majority of surgeons.[41]
Morphological features
Femtosecond laser can be programmed to cut from at least 100 μm below to 100 μm above the anterior capsule.[42] However, capsulotomy integrity is compromised by its inherent architecture of postage-stamp like perforations and additional aberrant pulses, possibly because of movements during eye fixation [Fig. 6]. Studies have been conducted to assess the centration, strength, and circularity parameters of manual versus lasered capsulotomy [Table 2].[17,38,43] Earlier studies showed that femtosecond created capsulotomies were more precise and strong than those created with the conventional manual technique.[40,41] However Abell et al.[43] reported that the rate of anterior capsule tears was significantly higher in femtosecond laser group (15/804 [1.87%]) than in manual surgery group (1/822 [0.12%]). Harthi et al. compared the scanning electron microscopic (SEM) features of the anterior capsule edge created by CCC and femtosecond capsulotomy and found that laser-assisted capsulotomies inherently have an irregular edge compared to the smooth edge of the manual CCC.[44] It appears logical for capsulorhexis marred by postage-stamp perforations to have less resistance to capsule tears than the smooth capsule edge.[43,44] Any irregularity at the edge of capsulotomy may act as focal point for the concentration of stress that would increase the probability of occurrence of anterior radial capsular tear.[45]
Figure 6.
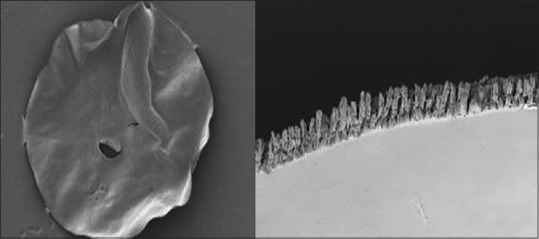
Scanning electron microscopy of femtosecond capsulotomy shows aberrant laser shots and a rough edge
Table 2.
Overview of various studies evaluating capsulotomy edges in different anterior capsulotomy techniques
| Type of capsulotomy | Author | Study type | Sample size | Capsulotomy edges |
|---|---|---|---|---|
| Manual CCC | Gimbel et al. 1990[9] | Prospective | 158 eyes | Strong capsular rim that resists tearing |
| Femto second laser | Abell et al. 2014[43] | Prospective cohort | 1626 eyes | Rough edge; postage stamp configuration |
| Femto second laser | Roberts et al. 2013[46] | Prospective interventional | 1500 eyes | Low rate of capsular tear |
| Femto second laser | Auffarth et al. 2013[47] | Experimental | Fresh pig eyes | Stronger anterior capsule opening than the standard manually performed capsulotomy. |
| Femto second laser | Kovács et al. 2014[48] | Prospective | 79 eyes | Low PCO |
| Femto second laser | Nagy et al. 2011[49] | Prospective | 111 eyes | Better overlap of capsular margins and better centration of IOL |
| PPC | Chang et al. 2016[53] | Human cadaver eyes and New Zealand white rabbits | 20 eyes | Smooth and regular |
| PPC | Hooshmand et al. 2018[58] | Prospective, multicenter case series | 100 eyes | Frayed edges |
| Comparison between CCC, Femtosecond laser, and PPC | Thompson et al. 2016[56] | A 3-arm study in paired human cadaver eyes | 44 eyes | PPC edge significantly stronger then Femotosecond laser and manual CCC |
PCO – Posterior capsular opacification; CCC – Central circular capsulorhexis
Complications
An increased rate of anterior capsule tears following femtosecond laser has been reported which remains an important concern.[41,43] Fixational eye movements during procedure can be a potential cause of compromised capsular integrity and postage-stamp perforations. This can lead to an increased rate of anterior capsule tears.
SEM of femtosecond lasered anterior capsule buttons demonstrated a rougher edge and scattered aberrant laser shots as opposed to manual CCC specimens and these might be postulated to predispose focal areas of anterior capsular rim to radial tears. Abell et al.[43] have suggested a relatively higher incidence of anterior capsule tears with femtosecond laser. Other case series have observed no difference, or the effect of a learning curve, with significant reduction of complication rates over time.[41,46] Worldwide experiences indicates a very low rate of anterior capsule tears after femtosecond-assisted capsulotomy.[46,47]
Kovács et al. have suggested that femtosecond capsulotomies reduce the incidence of PCO due to superior IOL positioning.[48,49] However it has been found to be associated with more incidences of capsular block syndrome.[50] It is hypothesized to produce intracapsular gas due to which sudden movement of lens nucleus during hydro dissection may block the fluid leaving no exit pathway for the flow, causing the rupture of PC.[50] Financial concerns along with mechanical factors and safety protocols should be considered before accepting it as the standard method of capsulotomy.[41,51]
Plasma blade capsulotomy
Plasma blade capsulotomy technique uses plasma technology to create a nearly resistance-free sharp incision into the anterior capsule under ophthalmic viscosurgical device (OVD). Delivered energy destroys the molecular structure of capsule, causes transient formation of microscopic plasma and cavitation bubbles in the tissue. It utilizes minimal power, causing no bleeding, or collateral tissue damage when done in well-formed AC.
Technique
Plasma blade includes an electrosurgical base unit attached to a Fugo blade tip. The blade filament is placed in contact with the capsule and the tip is to be moved in a circle at 360° while maintaining contact with the capsule with foot-pedal in use. Alternatively, multiple arcuate incisions are fashioned by blade tip that can be connected to form a circular capsulotomy. Main advantages of plasma blade capsulotomy technique are: (a) Prevention of peripheral extension of capsular tear as plasma blade can create a perpendicular capsular incision. (b) Neither a red reflex nor Trypan blue stain is needed to perform a capsulotomy. (c) It can safely create capsulotomy in a small pupil by ablating the anterior capsule behind the iris.
Morphological features
Although capsulotomies created by plasma blade do show some irregularities of the margins, they have been observed to be almost as smooth and regular as in the CCC under SEM evaluation.[52]
Complications
It has been reported that although the plasma blade can create a capsulotomy with good extensibility, it may lack the elastic strength present in the manual CCC due to presence of some margin irregularities.[52]
Precision Pulse Capsulotomy (PPC)/Zepto
Zepto capsulotomy system uses a nonlaser highly focused, fast, multipulse, low-energy discharge, which provides a reproducible, precisely automated, and fairly affordable technology to perform CCC. The device resembles a vitrectomy unit to create a capsulotomy independent of pupillary size, corneal clarity, or lens density.[53]
Technique
PPC is performed using a device with a soft collapsible tip and circular Nitinol cutting element that is connected to a control console.[54] Nitinol being superelastic allows the capsulotomy tip to be collapsed into a narrower and elongated shape for entry through a clear corneal incision of 2.2-2.4 mm. It later on re-expands automatically to its original circular shape within the AC. PPC system delivers a brief series of fast electrical pulses over 4 ms which prevents any heat dissipation beyond the Nitinol ring.[53] The tip is fully inserted into the AC maintained by OVD and is gently opposed to capsular surface without any undue pressure. Suction is applied through the suction cup which ensures uniform application of energy to anterior capsule. After anterior capsulotomy has been performed and subsequently suction is reversed automatically, the tip is taken out.
Zepto system has been observed to achieve a clean anterior capsulotomy in all kinds of cataracts and is especially useful for complicated cases with intumescent or brunescent lenses, zonulopathy, small pupils, and cases of cataract with corneal opacity.[54] Chang et al. reported PPC to be equally safe and with no greater zonular stress compared with CCC in human cadaver eyes.[53] PPC technique claims to have a short learning curve and success even in challenging cataract cases with reported consistent creation of a round, appropriately sized capsulotomy.[55]
Morphological features
Margins of anterior capsulotomy created by PPC are much smoother as compared to femtosecond laser as evidenced by human cadaver eye SEM studies [Fig. 7].[53]
Figure 7.
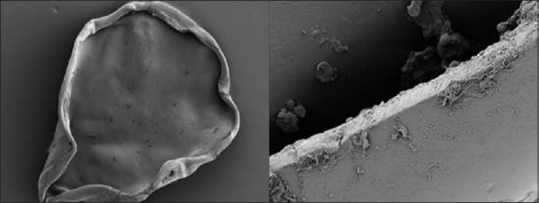
Scanning electron microscopy of precision pulse capsulotomy showing relatively smoother edge as compared to femtosecond laser-assisted capsulotomy
Thompson et al.[56] compared the anterior lens capsulotomy edge tear strength created by manual CCC, femtosecond laser capsulotomy, and automated Zepto device. The strength of PPC capsulotomy edge was found to be significantly stronger than that produced by femtosecond laser or manual CCC. This may be due to the rolled edges of the capsulotomy after the energy has been applied to the capsule [Fig. 7].
Complications
Hooshmand et al.[57] evaluated the clinical safety and performance of PPC device in 100 eyes having cataract surgery. They reported a high incidence of incomplete capsulotomy and radial tear rate that was possibly associated with the use of a dispersive OVD. They also found that edges of the capsulotomy were frayed when capsulotomy specimens were evaluated by SEM [Fig. 7] and by clinical examination of patients during postoperative period.[58]
Other capsulotomy techniques
Triangular capsulotomy: Vajpayee et al.[59] described a modified surgical technique of anterior capsulotomy for use in hypermature cataracts. Surgical steps include inferior linear capsulotomy with aspiration of milky cortex, inflation of the capsular sac with viscoelastic followed by reverse triangular anterior capsulotomy with Vannas scissors.
Crossed-swords, capsule-pinch technique: This technique is of special importance in soft cataract, elastic capsule, and deficient zonular support and works by puncturing the anterior capsule with a sharp needle or blade without creating any stress on zonules. Anterior capsule is pinched in between two 30-gauge needles until one of them penetrates to elevate a small flap which continues as CCC.[60]
Pulsed-electron avalanche knife: Palanker et al.[61] evaluated the pulsed-electron avalanche knife electrosurgical system as reported to create a histologically smooth cut.
Surgical devices: Verus ophthalmic calliper has a disposable single-use silicone ring inserted into the AC, positioned on the anterior capsule to guide capsulorhexis creation.[62]
Ring Calliper Designed by Tassignon et al. is effectual in achieving a perfectly sized capsulorhexis along with a perfectly centred one with reference to pupil and limbus.[63]
Dye assisted capsulotomy
Oblique illumination by operating microscope serves to present a fundal glow and allows visibility of anterior capsule during capsulotomy. However, capsular stains are commonly used to increase the capsular visibility in dense and hypermature cataracts. Common indications for dye-assisted capsulotomy are white cataracts,[64] dense cataracts,[64] cataracts with significant corneal haziness or scarring,[65] and during learning curve of phacoemulsification surgery.[66] Staining serves as an adjunct to enhance visualization of the anterior capsule especially in cases with impaired red reflex.
Trypan blue
Trypan blue 0.04–0.1% commercial ready to use dye has been utilized to enhance the visualization of anterior capsule during capsulotomy. Food and Drug Administration (FDA) has approved only Trypan blue for use as an adjunct to cataract surgery. It is contraindicated when a hydrophilic acrylic IOL is planned to be inserted into the eye because the dye may be absorbed by the IOL and stain it. Staining of the posterior lens capsule or vitreous face is generally self-limited, lasting up to 1 week.
The most common technique of staining remains injection of Trypan blue under an air bubble and subsequently washing the excess dye out after 10–15 sec.[64,67] Trypan blue has been reported to be effective in staining the anterior capsule with the highest safety profile.[68] However, the injections should be carefully administered to reduce diffusion towards posterior capsule and vitreous. Melles et al.[67] published study on use of 0.1% Trypan blue in a series of 30 patients with mature cataracts with no clinical evidence of increased inflammation, corneal endothelial impairment, or elevated IOP.
Many investigators have compared the efficacy of Trypan blue for capsular staining with other dyes. Dada et al.[69] studied capsular staining with five different agents - Trypan blue providing superior visualization of the capsule. Trypan blue as opposed to Indocyanine green (ICG) has been reported to provide significantly darker and more intense staining of the capsule, particularly useful in presence of complicating factors such as corneal edema.[65,70] In addition it greatly facilitates nuclear emulsification, in brunescent lens in particular because ICG does not provide adequate color contrast with this hue of nucleus.[70]
Studies have reported that Trypan blue may stiffen the anterior capsule and thus increase unwanted tears of capsulorhexis.[71,72] In addition, there are concerns that Trypan blue will negatively affect corneal endothelial cells and possibility of bacterial infection after injecting into the eye.[71]
ICG
It is used at concentrations of 0.125% to 0.5%, which is about 20 milliosmols hypotonic to aqueous humor. The intraocular administration of ICG is an off-label use of an FDA-approved product. ICG needs to be reconstituted to its appropriate concentration for intraocular use. Horiguchi et al.[73] first published a report on the use of 0.5% ICG to stain the anterior capsule on a randomized, prospective study of white cataracts with no statistically significant differences in laser flare-cell photometry, endothelial cell loss, or postoperative IOP spikes. Other studies have reported varied indications and outcomes of ICG capsular staining.[74]
Autologous blood
Use of autologous blood for capsular staining has been reported in various studies.[69] Autologous blood for intraocular use is prepared by using 2 mL of patient's blood, centrifuged at 2000 rpm for 2 min. Hemocoloration is done from the lowest layer of the centrifuged blood. However, autologous blood hemocoloration imparts poor visualization of the capsulorhexis and can cause extension of CCC.[69]
Fluorescein
Fluorescein 2% is an off-label use of FDA approved product, which has been utilized for capsular staining.[75] Fluorescein has been observed to cause poorer visualization of the capsulorhexis hence no longer used for capsular staining. Also, safety of intracameral fluorescein has always been a concern amongst cataract surgeons.
Gentian violet
Gentian violet has been used for staining anterior capsule and its efficacy in visualization of anterior capsule has been comparable to that of Trypan blue.[69] However, it can cause occurrence of adverse events like corneal edema.[76]
Capsulotomy in Special Circumstances
While CCC remains the gold standard for modern cataract surgery, its practice can be challenging in cases with zonular instability, poor pupillary dilation, corneal scarring with a hazy view, and hypermature cataracts associated with positive pressure on the capsule. Various modifications in technique of conventional CCC have been put forth for achieving central circular and adequately sized capsulotomy in such instances.
Capsulotomy in pediatric cataracts
Pediatric anterior lens capsule is thinner, stronger, and more elastic than in adults, necessitating precise surgical manoeuvres. Younger the child, greater is the elasticity of lens capsule. There has been evolving quest for different techniques to ensure a repeatable and reliable method of capsular opening in pediatric cases. Along with classical manual capsulorhexis, other methods which have been described are diathermy,[77] plasma blade,[78] vitrectorhexis,[79,80] and the two incision push-pull (TIPP) rhexes.[81] Modified TIPP technique was later put forth with advantages of being well-centered and optimum sized. Four arcuate incisions of 1–2 mm length are created with a cystitome. Subsequently, the resulted flaps are joined together to create an optimal capsulotomy.[82] With manual CCC the risk of peripheral extensions is more in children. Use of a high-molecular-weight OVD to flatten the anterior capsule is effective to keep the anterior capsule taut and counter the effects of low scleral rigidity and vitreous upthrust. Furthermore, construction of a slightly smaller than desired capsulotomy should be delibrated, because with the stretch in the anterior capsule, the opening would be larger at completion than it appears to be during active tearing. To avoid the difficulties with CCC in children, some surgeons prefer to perform can opener capsulotomy technique.[83,84] Wood and Schelonka[83] compared the strength and safety of a CCC with a can opener capsulotomy in a porcine model that closely resembles the high elasticity of the human pediatric lens capsule and concluded can opener capsulotomy to be better than a CCC. Titiyal et al. reported a technique of multiple postage stamp perforation capsulorhexis after lens aspiration and IOL implantation for paediatric cataracts.[85]
Vitrectorhexis - Anterior capsulotomy using a vitreous cutter is another widely used technique for anterior capsulotomy in pediatric cataracts and has been reported to be superior to conventional CCC in some studies.[80] Additional advantage of vitrectorhexis in paediatric eyes is that the anterior capsulotomy and the lens aspiration can be performed sequentially without taking out the instruments from the eye. For children with white cataracts and with fibrotic capsules use of radiofrequency diathermy and Fugo plasma blade have also been recommended.[78]
Capsulotomy in hypermature cataracts
In white intumescent cataracts, the capsule is more fragile in addition to impaired visibility of red reflex. Leakage of liquefied cortical material and capsulorhexis tears can extend to periphery because of high intralenticular pressure with sudden capsulorhexis radialization.[86]
In white hypermature cataracts, besides staining the anterior capsule to increase its visibility, creation of a small continuous curvilinear capsulorhexis, which is secondarily enlarged after aspirating the liquefied milky lens contents, can be attempted.[87] Kara-Junior et al.[86] evaluated two techniques of capsulorhexis for intumescent white cataracts: traditional one-stage continuous curvilinear capsulorhexis, and two-stage continuous curvilinear capsulorhexis. They concluded that two-stage continuous curvilinear capsulorhexis helps prevent sudden radialization of the CCC and other intraoperative complications. Femtosecond laser anterior capsulotomy technique has been described to be of special utility in white cataract, being accurate, circular, and centered thus avoiding IOL eccentricity and tilt caused by asymmetric capsular bag contraction.[88] Two-step femtosecond laser-assisted technique for intumescent white cataracts has been described with better 360° overlap of optic and implant stabilization.[89] Radiofrequency diathermy too has been successfully used to perform CCC in white cataracts with varied outcomes.[90]
Small pupil capsulotomy
Capsulorrhexis in cataracts with small pupil (pupil which has failed to dilate more than 4 mm) is associated with increased risk of complications. This is seen most commonly in patients suffering from pseudoexfoliation syndrome, posterior synechiae secondary to trauma, anterior uveitis, age-related iris sphincter sclerosis, diabetes, and pupillary fibrosis. Capsulotomy can be fashioned in such cases with the help of flexible hooks,[91] irrigating iris retractor,[92] excess OVDs usage,[93] and pupil dilating devices.[94] Recently, precision pulse capsulotomy technique has been used successfully to create capsulotomy in a case with small pupil.[95]
Cataract with corneal opacity
Presence of corneal haze or corneal opacity interferes with visualization of anterior capsule and subsequent capsulotomy which in turn significantly affects the outcome of cataract surgery. Trypan blue (0.1%) staining of anterior capsule aids in fashioning an intact circular capsulorhexis. In presence of corneal opacity, it functions by distinguishing the nonexcised, peripheral portion of the anterior capsule, from the gray lenticular mass underlying the excised, central portion of the capsule. Additionally, the stained peripheral rim remains clearly discernible during phacoemulsification, to facilitate subsequent steps. Bhartiya et al.[65] concluded that Trypan blue-assisted phacoemulsification is effective, both as a primary therapeutic option in cases where penetrating keratoplasty is not possible, and as an interim procedure in patients awaiting keratoplasty. Sinha et al.[96] have described a technique of performing pupillary sphincterotomy in cataracts with coexisting corneal opacity.
Endoillumination has been used to better visualize cataracts through corneal opacity during cataract surgery.[97] Endoilluminator when used as a light source outside cornea for CCC and inside the anterior chamber can facilitate various steps of phacoemulsification including performance of capsulotomy. Other techniques which have been described for performing capsulotomy in presence of corneal opacity with varied outcomes are use of intracameral dynamic spotlight, endoscope-aided, and Chandelier retroillumination-assisted.[98,99,100]
Future
The on-going pursuit for creating a perfectly sized central circular and strong capsulotomy has led researchers to assess other techniques with better outcomes. Important techniques under evaluation are
Capsulaser
The Capsulaser device (Excel-Len) is a continuous laser that is attached under the surgical microscope, connected to a small sized console. It is similar to femtosecond laser capsulotomy technique without the need for docking. The technique requires anterior capsule to be stained with Trypan blue, which creates a chromatically selective target for laser. Capsulotomies created by Capsulaser device have been documented to be well centered and without any late-onset contractions.
Aperture CTC
Continuous thermal capsulotomy (CTC) is a thermal device, which is currently under preclinical evaluation. It consists of a reusable hand piece and a disposable 1.2 mm diameter tip that houses a circular 5.25 mm filament. Tip is gently opposed to the capsule, to deliver a millisecond pulse of thermal energy, which melts the collagen and creates a perfectly round capsulotomy.
Conclusion
The technique of a circular continuous edge anterior capsulotomy is one of the most important steps toward a safer cataract procedure. The central circular smooth tear renders the anterior capsule its strength and a forgiving elasticity to withstand the subsequent manipulations of phacoemulsification. Techniques employed for anterior capsulotomy have undergone progressive evolution to achieve perfection in size, shape, centration, and overlap. Laser-assisted capsulotomy technique has been reported to have certain advantages over manual capsulotomy with reference to centration, size, and overlap; however, the higher cost of procedure remains the major limiting factor. While manual CCC remains the gold standard and most commonly utilized technique of anterior capsulotomy, different techniques can be utilized in varied circumstances as suited for patient needs and surgeon's comfort, for good visual and anatomical outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Steinert RF, Fine IH. 1st ed. Philadelphia: Saunders; 1995. Cataract Surgery: Technique, Complications and Management. [Google Scholar]
- 2.Kwitko M, Simcoe W. Manual extracapsular surgery. In: Marvin L Kwitko, Charles D Kelman., editors. The history of modern cataract surgery. The Netherlands: Hague: Kugler publishers; 1998. pp. 91–106. [Google Scholar]
- 3.Galand A. A simple method of implantation within the capsular bag. J Am Intraocul Implant Soc. 1983;9:330–2. doi: 10.1016/s0146-2776(83)80071-8. [DOI] [PubMed] [Google Scholar]
- 4.Haigh PM, Habib N, King AJW, David DB. Modified capsulorhexis vs. envelope capsulotomy in extracapsular cataract surgery. Eur J Implant Refract Surg. 1995;7:291–4. [Google Scholar]
- 5.Ndiaye PA, Eboulabeka E, Tchabi S, Ndiaye CS, Wane A, Ndiaye MR. Analysis of implant position after envelope technique in “white” cataract surgery. Dakar Med. 1999;44:16–9. [PubMed] [Google Scholar]
- 6.Akkin C, Ozler SA, Mentes J. Tilt and decentration of bag-fixated intraocular lenses: A comparative study between capsulorhexis and envelope techniques. Doc Ophthalmol. 1994;87:199–209. doi: 10.1007/BF01203850. [DOI] [PubMed] [Google Scholar]
- 7.Oner FH, Durak I, Soylev M, Ergin M. Long-term results of various anterior capsulotomies and radial tears on intraocular lens centration. Ophthalmic Surg Lasers. 2001;32:118–23. [PubMed] [Google Scholar]
- 8.Budo C, Montanus F. Small capsulotomy and great implant lens. Bull Soc Belge Ophtalmol. 1991;242:41–6. [PubMed] [Google Scholar]
- 9.Gimbel HV, Neuhann T. Development, advantages, and methods of the continuous circular capsulorhexis technique. J Cataract Refract Surg. 1990;16:31–7. doi: 10.1016/s0886-3350(13)80870-x. [DOI] [PubMed] [Google Scholar]
- 10.Gimbel HV, Neuhann T. Continuous curvilinear capsulorhexis. J Cataract Refract Surg. 1991;17:110–1. doi: 10.1016/s0886-3350(13)81001-2. [DOI] [PubMed] [Google Scholar]
- 11.Neuhann T. Theorie und operations technik der kapsulorhexis. Klin Monatsbl Augenheilkd. 1987;190:542–5. doi: 10.1055/s-2008-1050454. [DOI] [PubMed] [Google Scholar]
- 12.Arshinoff S. Mechanics of capsulorhexis. J Cataract Refract Surg. 1992;18:623–8. doi: 10.1016/s0886-3350(13)80456-7. [DOI] [PubMed] [Google Scholar]
- 13.Dada T, Sethi H. Forceps capsulorhexis. J Cataract Refract Surg. 2002;28:1491. doi: 10.1016/s0886-3350(02)01478-5. [DOI] [PubMed] [Google Scholar]
- 14.Malik KPS, Goel R. Bimanual capsulorrhexis using Sinskey hook. Contact lens & anterior eye. J Br Contact Lens Assoc. 2012;35:228–9. doi: 10.1016/j.clae.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Gimbel HV. Two staged capsulorhexis for endocapsular phacoemulsification. J Cataract Refract Surg. 1990;16:246–9. doi: 10.1016/s0886-3350(13)80739-0. [DOI] [PubMed] [Google Scholar]
- 16.Powers MA, Kahook MY. New device for creating a continuous curvilinear capsulorhexis. J Cataract Refract Surg. 2014;40:822–30. doi: 10.1016/j.jcrs.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 17.Kranitz K, Takacs A, Mihaltz K. Femtosecond laser capsulotomy and manual continuous curvilinear capsulorrhexis parameters and their effects on intraocular lens centration. J Refract Surg. 2011;27:558–63. doi: 10.3928/1081597X-20110623-03. [DOI] [PubMed] [Google Scholar]
- 18.Hollick EJ, Spalton DJ, Meacock WR. The effect of capsulorrhexis size on posterior capsular opacification: One-year results of a randomized prospective trial. Am J Ophthalmol. 1999;128:271–9. doi: 10.1016/s0002-9394(99)00157-9. [DOI] [PubMed] [Google Scholar]
- 19.Aykan U, Bilge AH, Karadayi K, Akin T. The effect of capsulorhexis size on development of posterior capsule opacification: Small (4.5 to 5.0 mm) versus large (6.0 to 7.0 mm) Eur J Ophthalmol. 2003;13:541–5. doi: 10.1177/112067210301300606. [DOI] [PubMed] [Google Scholar]
- 20.Ravalico G, Tognetto D, Palomba M. Capsulorhexis size and posterior capsule opacification. J Cataract Refract Surg. 1996;22:98–103. doi: 10.1016/s0886-3350(96)80277-x. [DOI] [PubMed] [Google Scholar]
- 21.Cekiç O, Batman C. The relationship between capsulorhexis size and anterior chamber depth relation. Ophthalmic Surg Lasers. 1999;30:185–90. [PubMed] [Google Scholar]
- 22.Wirtitsch MG, Findl O, Menapace R, Kriechbaum K, Koeppl C, Buehl W, et al. Effect of haptic design on change in axial lens position after cataract surgery. J Cataract Refract Surg. 2004;30:45–51. doi: 10.1016/S0886-3350(03)00459-0. [DOI] [PubMed] [Google Scholar]
- 23.Henderson BA, Yi DH, Constantine JB, Geneva II. New preventative approach for negative dysphotopsia. J Cataract Refract Surg. 2016;42:1449–55. doi: 10.1016/j.jcrs.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Okada M, Hersh D, Paul E, van der Straaten D. Effect of centration and circularity of manual capsulorrhexis on cataract surgery refractive outcomes. Ophthalmology. 2014;121:763–70. doi: 10.1016/j.ophtha.2013.09.049. [DOI] [PubMed] [Google Scholar]
- 25.Joo CK, Shin Ja, Kim JH. Capsular opening contraction after continuous curvilinear capsulorhexis and intraocular lens implantation. J Cataract Refract Surg. 1996;22:585–90. doi: 10.1016/s0886-3350(96)80014-9. [DOI] [PubMed] [Google Scholar]
- 26.Tan X, Liu Z, Zhu Y, Chen C, Huang S, Chen B. Fate of in situ lens regeneration is determined by capsulorhexis size. Curr Mol Med. 2017;17:270–9. doi: 10.2174/1566524017666171106110304. [DOI] [PubMed] [Google Scholar]
- 27.Olali CA, Ahmed S, Gupta M. Surgical outcome following breach rhexis. Eur J Ophthalmol. 2007;17:565–70. doi: 10.1177/112067210701700414. [DOI] [PubMed] [Google Scholar]
- 28.Marques FF, Marques DMV, Osher RH, Osher JM. Fate of anterior capsule tears during cataract surgery. J Cataract Refract Surg. 2006;32:1638–42. doi: 10.1016/j.jcrs.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Carifi G, Miller MH, Pitsas C, Zygoura V, Deshmukh RR, Kopsachilis N. Complications and outcomes of phacoemulsification cataract surgery complicated by anterior capsule tear. Am J Ophthalmol. 2015;159:463–9. doi: 10.1016/j.ajo.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 30.Mohammadpour M. Rescue of an extending capsulorhexis by creating a midway tangential anterior capsular flap: A novel technique in 22 eyes. Can J Ophthalmol. 2010;45:256–8. doi: 10.3129/i09-260. [DOI] [PubMed] [Google Scholar]
- 31.Kránitz K, Miháltz K, Sándor GL. Intraocular lens tilt and decentration measured by Scheimpflug camera following manual or femtosecond laser-created continuous circular capsulotomy. J Refract Surg. 2012;28:259–63. doi: 10.3928/1081597X-20120309-01. [DOI] [PubMed] [Google Scholar]
- 32.Little BC, Smith JH, Packer M. Little capsulorhexis tear-out rescue. J Cataract Refract Surg. 2006;32:1420–2. doi: 10.1016/j.jcrs.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 33.Karim SMR, Ong CT, Miah MR, Sleep T, Hanifudin A. A novel technique of rescuing capsulorhexis radial tear-out using a cystotome. J Vis Exp. 2011;47:2317. doi: 10.3791/2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Waard PWT, Budo CJ, Melles GRJ. Trypan blue capsular staining to find the leading edge of a lost capsulorhexis. AmJ Ophthalmol. 2002;134:271–2. doi: 10.1016/s0002-9394(02)01455-1. [DOI] [PubMed] [Google Scholar]
- 35.Page TP. Anterior zonulotomy: Rescue technique for capsulorhexis tear-out. J Cataract Refract Surg. 2015;41:2036–9. doi: 10.1016/j.jcrs.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Franchini A. The rhexis: Errant, compromised, or smaller or larger than planned. Cataract Refract Surg Today. 2012;3:35–8. [Google Scholar]
- 37.Friedman NJ, Palanker DV, Schuele G. Femtosecond laser capsulotomy. J Cataract Refract Surg. 2011;37:1189–98. doi: 10.1016/j.jcrs.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 38.Nagy Z, Takacs A, Filkorn T. Initial clinical evaluation of an intraocular femtosecond laser in cataract surgery. J Refract Surg. 2009;25:1053–6. doi: 10.3928/1081597X-20091117-04. [DOI] [PubMed] [Google Scholar]
- 39.Naranjo-Tackman R. How a femtosecond laser increases safety and precision in cataract surgery? Curr Opin Ophthalmol. 2011;22:53–7. doi: 10.1097/ICU.0b013e3283415026. [DOI] [PubMed] [Google Scholar]
- 40.Packer M, Teuma EV, Glasser A, Bott S. Defining the ideal femtosecond laser capsulotomy. Br J Ophthalmol. 2015;99:1137–42. doi: 10.1136/bjophthalmol-2014-306065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang JS, Chen IN, Chan WM, Ng JC, Chan VK, Law AK. Initial evaluation of a femtosecond laser system in cataract surgery. J Cataract Refract Surg. 2014;40:29–36. doi: 10.1016/j.jcrs.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 42.Dilraj S, Grewal, Schultz T, Basti S, Burkhard D. Femtosecond laserassisted cataract surgery-current status and future directions. Surv Ophthalmol. 2016;61:103–31. doi: 10.1016/j.survophthal.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Abell RG, Davies PE, Phelan D, Goemann K, McPherson ZE, Vote BJ. Anterior capsulotomy integrity after femtosecond laser-assisted cataract surgery. Ophthalmology. 2014;121:17–24. doi: 10.1016/j.ophtha.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 44.Harthi KA, Shahwan S, Towerki A, Banerjee P. Comparison of the anterior capsulotomy edge created by manual capsulorhexis and 2 femtosecond laser platforms: Scanning electron microscopy study. J Cataract Refract Surg. 2014;12:2106–12. doi: 10.1016/j.jcrs.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Morgan JE, Ellingham RB, Young RD, Trmal GJ. The mechanical properties of the human lens capsule following capsulorrhexis or radiofrequency diathermy capsulotomy. Arch Ophthalmol. 1996;114:1110–5. doi: 10.1001/archopht.1996.01100140312010. [DOI] [PubMed] [Google Scholar]
- 46.Roberts TV, Lawless M, Bali SJ. Surgical outcomes and safety of femtosecond laser cataract surgery: A prospective study of 1500 consecutive cases. Ophthalmology. 2013;120:227–33. doi: 10.1016/j.ophtha.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 47.Auffarth GU, Reddy KP, Ritter R. Comparison of the maximum applicable stretch force after femtosecond laser-assisted and manual anterior capsulotomy. J Cataract Refract Surg. 2013;39:105–8. doi: 10.1016/j.jcrs.2012.08.065. [DOI] [PubMed] [Google Scholar]
- 48.Kovács I, Kránitz K, Sándor GL. The effect of femtosecond laser capsulotomy on the development of posterior capsule opacification. J Refract Surg. 2014;30:154–8. doi: 10.3928/1081597X-20140217-01. [DOI] [PubMed] [Google Scholar]
- 49.Nagy ZZ, Kranitz K, Takacs AI, Mihaltz K, Kovács I, Knorz MC. Comparison of intraocular lens decentration parameters after femtosecond and manual capsulotomies. J Refract Surg. 2011;27:564–9. doi: 10.3928/1081597X-20110607-01. [DOI] [PubMed] [Google Scholar]
- 50.Roberts TV, Sutton G, Lawless MA. Capsular block syndrome associated with femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2011;37:2068–70. doi: 10.1016/j.jcrs.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Hatch KM, Talamo JH. Laser-assisted cataract surgery: Benefits and barriers. Curr Opin Ophthalmol. 2014;25:54–61. doi: 10.1097/ICU.0000000000000013. [DOI] [PubMed] [Google Scholar]
- 52.Izak AM, Werner L, Pandey SK, Apple DJ, Izak GJ. Analysis of the capsule edge after Fugo plasma blade capsulotomy, continuous curvilinear capsulorhexis, and can-opener capsulotomy. J Cataract Refract Surg. 2004;30:2606–11. doi: 10.1016/j.jcrs.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 53.Chang DF, Mammalis N. Precision pulse capsulotomy preclinical safety and performance of a new capsulotomy technology. Ophthalmology. 2016;123:255–64. doi: 10.1016/j.ophtha.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 54.Chang DF. Zepto precision pulse capsulotomy: A new automated and disposable capsulotomy technology. Indian J Ophthalmol. 2017;65:1411–4. doi: 10.4103/ijo.IJO_737_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waltz K, Thompson VM, Quesada G. Precision pulse capsulotomy: Initial clinical experience in simple and challenging cataract surgery cases. J Cataract Refract Surg. 2017;43:606–14. doi: 10.1016/j.jcrs.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 56.Thompson VM, Berdahl JP, Solano JM, Chang DF. Comparison of manual, femtosecond laser, and precision pulse capsulotomy edge tear strength in paired human cadaver eyes. Ophthalmology. 2016;123:265–74. doi: 10.1016/j.ophtha.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 57.Hooshmand J, Abell RG, Allen P, Vote BJ. Thermal capsulotomy: Initial clinical experience, intraoperative performance, safety, and early postoperative outcomes of precision pulse capsulotomy technology. J Cataract Refract Surg. 2018;44:355–61. doi: 10.1016/j.jcrs.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 58.Hooshmand J, Abell RG, Goemann K, Davies PEJ, Vote BJ. Ultrastructural integrity of human capsulotomies created by a thermal device. Ophthalmology. 2018;125:340–4. doi: 10.1016/j.ophtha.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 59.Vajpayee RB, Angra SK, Honavar SG, Katoch S, Prasad N, Bansal A, et al. Capsulotomy for phacoemulsification in hypermature cataracts. J Cataract Refract Surg. 1995;21:612–5. doi: 10.1016/s0886-3350(13)80554-8. [DOI] [PubMed] [Google Scholar]
- 60.Snyder ME, Lindsell LB. Crossed-swords, capsule-pinch technique for capsulotomy in pediatric and/or loose lens cataract extraction. J Cat Refract Surg. 2010;36:197–9. doi: 10.1016/j.jcrs.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 61.Palanker D, Nomoto H, Huie P, Vankov A, Chang D. Anterior capsulotomy with a pulsed electron avalanche knife. J Cataract Refract Surg. 2010;36:127–32. doi: 10.1016/j.jcrs.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kahook MY, Cionni RJ, Taravella MJ, Ang RE, Waite AN, Solomon JD, et al. Continuous curvilinear capsulorhexis performed with the VERUS ophthalmic caliper. J Refract Surg. 2016;32:654–8. doi: 10.3928/1081597X-20160609-02. [DOI] [PubMed] [Google Scholar]
- 63.Tassignon M-J, Rozema JJ, Gobin L. Ring-shaped caliper for better anterior capsulorhexis sizing and centration. J Cataract Refract Surg. 2006;32:1253–5. doi: 10.1016/j.jcrs.2006.02.067. [DOI] [PubMed] [Google Scholar]
- 64.Pandey SK, Werner L, Escobar-Gomez M, Roig-Melo EA, Apple DJ. Dye enhanced cataract surgery. Anterior capsule staining for capsulorhexis in advanced/white cataract. J Cataract Refract Surg. 2000;26:1052–9. doi: 10.1016/s0886-3350(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 65.Bhartiya P, Sharma N, Ray M, Sinha R, Vajpayee RB. Trypan blue assisted phacoemulsification in corneal opacities. Br J Ophthalmol. 2002;86:857–9. doi: 10.1136/bjo.86.8.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dada T, Ray M, Bhartiya P, Vajpayee RB. Trypan-blue-assisted capsulorhexis for trainee phacoemulsification surgeons. J Cataract Refract Surg. 2002;28:575–6. doi: 10.1016/s0886-3350(02)01316-0. [DOI] [PubMed] [Google Scholar]
- 67.Melles GR, de Waard PW, Pameyer JH. Trypan blue capsule staining to visualize the capsulorhexis in cataract surgery. J Cataract Refract Surg. 1999;25:7–13. doi: 10.1016/s0886-3350(99)80004-2. [DOI] [PubMed] [Google Scholar]
- 68.Ozturk F, Osher RH. Capsular staining: Recent developments. Curr Opin Ophthalmol. 2006;17:42–4. doi: 10.1097/01.icu.0000193066.09499.a5. [DOI] [PubMed] [Google Scholar]
- 69.Dada VK, Sharma N, Sudan R, Sethi H, Dada T, Pangtey MS. Anterior capsule staining for capsulorhexis in cases of white cataract: Comparative clinical study. J Cataract Refract Surg. 2004;30:326–33. doi: 10.1016/S0886-3350(03)00573-X. [DOI] [PubMed] [Google Scholar]
- 70.Kothari K, Jain SS, Shah NJ. Anterior capsular staining with trypan blue for capsulorhexis in mature and hypermature cataracts; a preliminary study. Indian J Ophthalmol. 2001;49:177–80. [PubMed] [Google Scholar]
- 71.Wollensak G, Spörl E, Pham DT. Biomechanical changes in the anterior lens capsule after trypan blue staining. J Cataract Refract Surg. 2004;30:1526–30. doi: 10.1016/j.jcrs.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 72.Dick HB, Aliyeva SE, Hengerer F. Effect of trypan blue on the elasticity of the human anterior lens capsule. J Cataract Refract Surg. 2008;34:1367–73. doi: 10.1016/j.jcrs.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 73.Horiguchi M, Miyake K, Ohta I, Ito Y. Staining of the lens capsule for circular continuous capsulorhexis in eyes with white cataract. Arch Ophthalmol. 1998;116:535–7. doi: 10.1001/archopht.116.4.535. [DOI] [PubMed] [Google Scholar]
- 74.Guo S, Caputo A, Wagner R, DeRespinis P. Enhanced visualization of capsulorhexis with indocyanine green staining in pediatric white cataracts. J Pediatr Ophthalmol Strabismus. 2003;40:268–71. doi: 10.3928/0191-3913-20030901-06. [DOI] [PubMed] [Google Scholar]
- 75.Nahra D, Castilla M. Fluorescein-stained capsulorrhexis. J Cataract Refract Surg. 1998;24:1169–70. doi: 10.1016/s0886-3350(98)80002-3. [DOI] [PubMed] [Google Scholar]
- 76.Unlü K, Askünger A, Söker S, Kilinç N, Karaca C, Erdinc M. Gentian violetsolution for staining the anterior capsule. J Cataract Refract Surg. 2000;26:1228–32. doi: 10.1016/s0886-3350(00)00360-6. [DOI] [PubMed] [Google Scholar]
- 77.Comer RM, Abdulla N, O’Keefe M. Radiofrequency diathermy capsulorhexis of the anterior and posterior capsules in pediatric cataract surgery: Preliminary results. J Cataract Refract Surg. 1997;23:641–4. doi: 10.1016/s0886-3350(97)80047-8. [DOI] [PubMed] [Google Scholar]
- 78.Singh D. Use of the Fugo blade in complicated cases. J Cataract Refract Surg. 2002;28:573–4. doi: 10.1016/s0886-3350(02)01314-7. [DOI] [PubMed] [Google Scholar]
- 79.Luo L, Lin H, Chen W, Wang C, Zhang X, Tang X. In-the-bag intraocular lens placement via secondary capsulorhexis with radiofrequency diathermy in pediatric aphakic eyes. PLoS One. 2013;8:e62381. doi: 10.1371/journal.pone.0062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andreo LK, Wilson ME, Apple DJ. Elastic properties and scanning electron microscopic appearance of manual continuous curvilinear capsulorhexis and vitrectorhexis in an animal model of pediatric cataract. J Cataract Refract Surg. 1999;25:534–9. doi: 10.1016/s0886-3350(99)80051-0. [DOI] [PubMed] [Google Scholar]
- 81.Nischal KK. Two-incision push-pull capsulorhexis for pediatric cataract surgery. J Cataract Refract Surg. 2002;28:593–5. doi: 10.1016/s0886-3350(01)01125-7. [DOI] [PubMed] [Google Scholar]
- 82.Mohammadpour M. Four-incision capsulorhexis in pediatric cataract surgery. J Cataract Refract Surg. 2007;33:1155–7. doi: 10.1016/j.jcrs.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 83.Wood MG, Schelonka LP. A porcine model predicts that a can-opener capsulotomy can be done safely in pediatric patients. J AAPOS. 1999;3:356–62. doi: 10.1016/s1091-8531(99)70045-5. [DOI] [PubMed] [Google Scholar]
- 84.Gimbel HV, DeBroff BM. Surgical management of pediatric cataracts. In: Steinert RF, editor. Cataract Surgery–Technique, Complications, Management. St Louis: Saunders; 2004. pp. 273–93. [Google Scholar]
- 85.Titiyal JS, Sinha R, Sharma N, Vajpayee RB. Postage stamp multiple anterior capsulorhexisotomies in pediatric cataract surgery. BMC Ophthalmol. 2005;5:3. doi: 10.1186/1471-2415-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kara-Junior N, de Santhiago MR, Kawakami A, Carricondo P, Hida WT. Mini rhexis for white intumescent cataracts. Clinics (Sao Paulo) 2009;64:309–12. doi: 10.1590/S1807-59322009000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gimbel HV, Willerscheidt AB. What to do with limited view: The intumescent cataract. J Cataract Refract Surg. 1993;19:657–61. doi: 10.1016/s0886-3350(13)80021-1. [DOI] [PubMed] [Google Scholar]
- 88.Peng TT, Wang Y, Bao XY. Preliminary report on the application of femtosecond laser-assisted anterior capsulotomy in intumescent white cataract surgery. Zhonghua Yan Ke Za Zhi. 2017;53:281–7. doi: 10.3760/cma.j.issn.0412-4081.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 89.Schultz T, Dick HB. Laser-assisted mini-capsulotomy: A new technique for intumescent white cataracts. J Refract Surg. 2014;30:742–5. doi: 10.3928/1081597X-20141021-05. [DOI] [PubMed] [Google Scholar]
- 90.Pham DT, Liekfeld A, Hartmann C. Capsulotomy in intumescent cataract with the high frequency diathermy capsulotomy. Klin Monbl Augenheilkd. 1998;212:29–31. doi: 10.1055/s-2008-1034827. [DOI] [PubMed] [Google Scholar]
- 91.Smith GT, Liu CS. Flexible iris hooks for phacoemulsification in patients with iridoschisis. J Cataract Refract Surg. 2000;26:1277–80. doi: 10.1016/s0886-3350(00)00523-x. [DOI] [PubMed] [Google Scholar]
- 92.Bohm P, Horvath J, Zahorcova M. Irrigating iris retractor for complicated cataract surgery. J Cataract Refract Surg. 2009;35:419–21. doi: 10.1016/j.jcrs.2008.10.056. [DOI] [PubMed] [Google Scholar]
- 93.Jhanji V, Sharma N, Vajpayee RB. Management of intraoperative miosis during pediatric cataract surgery using Healon 5. Middle East Afr J Ophthalmol. 2011;18:55–7. doi: 10.4103/0974-9233.75888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang DF. Use of Malyugin pupil expansion device for intraoperative floppy-iris syndrome: Results in 30 consecutive cases. J Cataract Refract Surg. 2008;34:835–41. doi: 10.1016/j.jcrs.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 95.Pandey SK, Sharma V. Zepto rhexis-A new surgical technique of capsulorhexis using precision nano-pulse technology in difficult cataract cases. Indian J Ophthalmol. 2018;66:1165–8. doi: 10.4103/ijo.IJO_1006_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sinha R, Sharma N, Vajpayee RB. Visual outcome of cataract surgery with pupillary sphincterotomy in eyes with coexisting corneal opacity. BMC Med. 2004;2:10. doi: 10.1186/1741-7015-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nishimura A, Kobayashi A, Segawa Y, Sugiyama K. Endoillumination-assisted cataract surgery in a patient with corneal opacity. J Cataract Refract Surg. 2003;29:2277–80. doi: 10.1016/s0886-3350(03)00493-0. [DOI] [PubMed] [Google Scholar]
- 98.Moon H, Lee JH, Lee JY, Kim KH, Lee DY. Intracameral dynamic spotlight-assisted cataract surgery in eyes with corneal opacity, small pupil or advanced cataract. Acta Ophthalmol. 2015;93:388–90. doi: 10.1111/aos.12428. [DOI] [PubMed] [Google Scholar]
- 99.Uka J, Minamoto A, Hirayama T, Adachi T, Yamane K, Mishima HK. Endoscope-aided cataract surgery in corneal opacity associated with aniridia. J Cataract Refract Surg. 2005;31:1455–6. doi: 10.1016/j.jcrs.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 100.Oshima Y, Shima C, Maeda N, Tano YJ. Chandelier retro illumination assisted torsional oscillation for cataract surgery in patients with severe corneal opacity. Cataract Refract Surg. 2007;33:2018–22. doi: 10.1016/j.jcrs.2007.07.055. [DOI] [PubMed] [Google Scholar]