Figure 2.
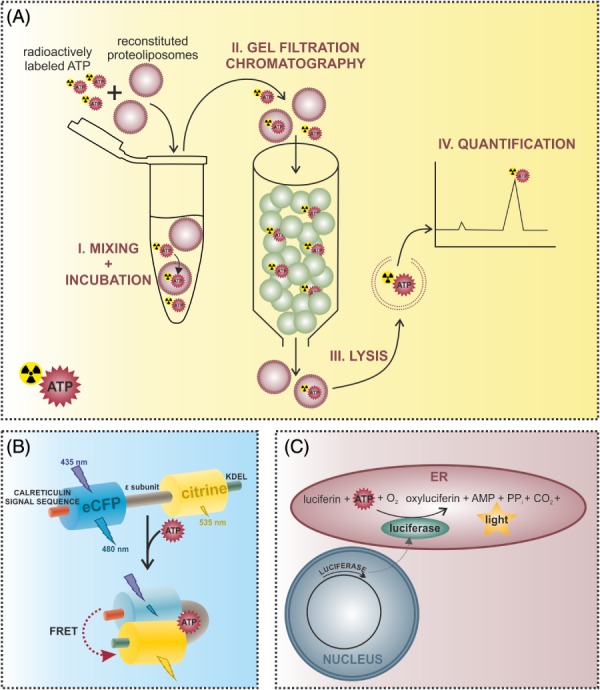
Methods for the investigation of endoplasmic reticulum (ER) ATP dynamics. (A) ATP transport into ER‐derived vesicles or reconstituted liposomes containing ER proteins can be measured using an in vitro approach. Proteoliposomes are incubated with radioactively labeled ATP and then applied to a gel filtration column. Free ATP binds to the matrix, while proteoliposomes and incorporated ATP are eluted in the void volume. Proteoliposomes are then lysed to determine the amount of imported ATP via high‐performance liquid chromatography (HPLC) or liquid scintillation counting. (B) Alterations of ER ATP levels can be measured using genetically encoded fluorescent ATP sensors targeted to the ER. The ERAT4.01 probe for example is a Förster/fluorescence resonance energy transfer (FRET)‐based sensor consisting of two fluorescent proteins – an enhanced cyan fluorescent protein (CFP) and the yellow fluorescent protein (YFP) variant citrine – flanking the ATP binding ϵ‐subunit of the FoF1‐ATP synthase from Bacillus subtilis (Imamura et al., 2009; Vishnu et al., 2014). The N‐terminal fusion of the calreticulin signal sequence and the C‐terminal addition of the ER retention signal with the amino acid sequence KDEL (lysine–aspartic acid–glutamic acid–leucine) allow the efficient targeting of the probe to the ER. Binding of ATP to the ϵ‐subunit causes a conformational change of the sensor, which increases the FRET signal intensity. (C) Alternatively, ER ATP levels can be determined using an ER‐targeted heterologously expressed firefly luciferase, which catalyses the reaction of oxyluciferin to luciferin, resulting in light emission. Since this reaction is ATP dependent, the intensity of the emitted light is proportional to the ATP concentration.
