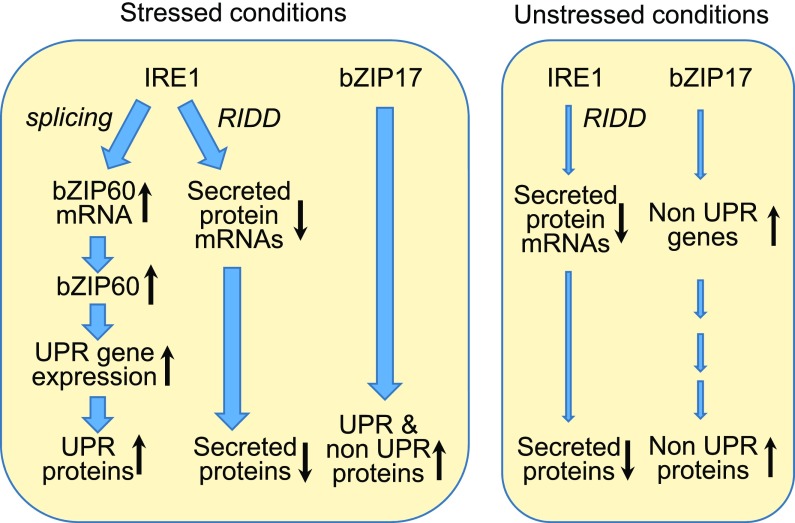
Figure 7.
Proposed model for the function of IRE1 and bZIP17 under stressed and unstressed conditions. Under stressed conditions, IRE1 up-regulates the expression of the canonical UPR genes in a bZIP60-dependent manner. Under these conditions, the RIDD activity of IRE1 is also activated, leading to the degradation of the RNAs of many secreted protein genes. In addition, bZIP17 is mobilized and functions along with bZIP60 to up-regulate canonical and noncanonical UPR genes. Under unstressed conditions, the RIDD activity of IRE1 presumably leads to the degradation of unidentified mRNAs, but likely those encoding secreted protein. bZIP17 is also modestly active, leading to the production of some noncanonical UPR proteins.