The degradation of branched-chain amino acids provides carbon precursors for lipid biosynthesis during nitrogen starvation and ATP for lipid remobilization upon nitrogen resupply in green algae.
Abstract
Nitrogen (N) starvation-induced triacylglycerol (TAG) synthesis, and its complex relationship with starch metabolism in algal cells, has been intensively studied; however, few studies have examined the interaction between amino acid metabolism and TAG biosynthesis. Here, via a forward genetic screen for TAG homeostasis, we isolated a Chlamydomonas (Chlamydomonas reinhardtii) mutant (bkdE1α) that is deficient in the E1α subunit of the branched-chain ketoacid dehydrogenase (BCKDH) complex. Metabolomics analysis revealed a defect in the catabolism of branched-chain amino acids in bkdE1α. Furthermore, this mutant accumulated 30% less TAG than the parental strain during N starvation and was compromised in TAG remobilization upon N resupply. Intriguingly, the rate of mitochondrial respiration was 20% to 35% lower in bkdE1α compared with the parental strains. Three additional knockout mutants of the other components of the BCKDH complex exhibited phenotypes similar to that of bkdE1α. Transcriptional responses of BCKDH to different N status were consistent with its role in TAG homeostasis. Collectively, these results indicate that branched-chain amino acid catabolism contributes to TAG metabolism by providing carbon precursors and ATP, thus highlighting the complex interplay between distinct subcellular metabolisms for oil storage in green microalgae.
Microalgae are fast-growing microorganisms that have developed efficient mechanisms to harvest and transform solar energy into energy-rich molecules such as lipids. They are thus promising cell factories for the production of fuels and biomaterials for chemical industries. However, several fundamental as well as engineering challenges need to be resolved before the establishment of a sector on algal bioenergy. A major challenge is that in algal cells, significant oil accumulation occurs only under conditions when growth is impaired (such as nitrogen [N] deficiency, high salinity, stationary phase, or high light; Wang et al., 2009; Moellering and Benning, 2010; Siaut et al., 2011; Urzica et al., 2013; Goold et al., 2016). To uncouple the inverse relationship between triacylglycerol (TAG) synthesis and cell division (i.e. biomass growth), a deeper and holistic understanding of the pathways for fatty acid synthesis and their assembly into oil (i.e. TAG), as well as the regulatory mechanisms involved, is required.
N starvation-induced oil accumulation in algal cells has been mostly studied through omics studies, as well as the enzymatic steps and regulations involved (Work et al., 2010; Boyle et al., 2012; Chen and Smith, 2012; Li et al., 2012; Schmollinger et al., 2014; Tsai et al., 2014; Kajikawa et al., 2015; Warakanont et al., 2015; Schulz-Raffelt et al., 2016; Kong et al., 2017). Studies on the carbon and energy sources required are more scarce and have mostly focused on competition with starch accumulation for carbon precursors (Wang et al., 2009; Li et al., 2010; Work et al., 2010; Siaut et al., 2011; Krishnan et al., 2015). Increasing evidence in plants suggests that the control of TAG synthesis occurs at the earlier step of de novo fatty acid synthesis (Bourgis et al., 2011). A positive correlation between the rate of de novo fatty acid synthesis and the amount of carbon precursors has been found in both plants and algae (Fan et al., 2012; Ramanan et al., 2013; Goodenough et al., 2014; Avidan et al., 2015). N-starved cells are known to overaccumulate acetyl-CoA prior to TAG synthesis in the green alga Chlorella desiccata (Avidan et al., 2015). It has also been observed that feeding cells with an additional amount of acetate (an acetate boost) enhances lipid synthesis in the model microalga Chlamydomonas (Chlamydomonas reinhardtii; Goodson et al., 2011; Fan et al., 2012), which highlights the importance of acetyl-CoA supply as a carbon source for lipid synthesis.
In addition to increased de novo fatty acid synthesis, membrane lipids are another source of acyl chains for TAG assembly during N starvation (Fan et al., 2011). This is supported by transcriptional, biochemical, and genetic evidence. First, many genes encoding lipolytic enzymes were up-regulated upon N starvation (Miller et al., 2010). Second, the degradation of major membrane lipids occurs simultaneously to TAG accumulation (Moellering and Benning, 2010; Siaut et al., 2011). Finally, the pgd1 mutant, deficient in a major galactolipid lipase, Plastid Galactoglycerolipid Degradation1 (PGD1), made less TAG than its parental strain, providing a compelling demonstration of the flux of acyl chains from plastid lipid to TAG (Li et al., 2012). Furthermore, the result obtained from the study of the pgd1 mutant could also indicate that de novo synthesized fatty acids, at least partly, first incorporated into plastid lipids before entering TAG synthesis.
Besides carbon precursors, lipid synthesis requires a stoichiometric supply of ATP and reducing equivalents NADPH in a ratio of 1:2 (Ohlrogge and Browse, 1995; Li-Beisson et al., 2013). The roles of both energetic and redox considerations in governing subcellular metabolism have been frequently demonstrated (Geigenberger et al., 2005; Michelet et al., 2013; Kong et al., 2018a). However, little is known concerning the sources and variations of ATP supply on lipid synthesis.
Alongside lipid and starch, amino acids (AA) are known respiratory substrates (Araújo et al., 2010; Binder, 2010; Kochevenko et al., 2012; Hildebrandt et al., 2015). Among all AAs synthesized by plants and green algae, Leu, Ile, and Val have in common a branched aliphatic chain and their degradation products include an acetyl-CoA, potential substrates for de novo fatty acid synthesis (Binder, 2010). These three AAs are collectively called branched-chain amino acids (BCAAs). In addition to acting as a respiratory substrate, BCAAs also play a structural and signaling role (Kimball and Jefferson, 2006; Binder, 2010). Thus far, the relationship between BCAA catabolism and lipid synthesis has been studied in mammals (Green et al., 2016), the model diatom Phaeodactylum tricornutum (Ge et al., 2014), and more recently in Dunaliella tertiolecta, where Yao et al. (2017) reported that acetyl-CoAs produced through BCAA catabolism contributed to TAG synthesis. However, this interaction has not been investigated at the molecular level in the green lineage, notably plants or green algae, because the genetic basis of the mutation in the mutant strain of D. tertiolecta has not been reported. Moreover, the connection between BCAA and lipid is currently limited to the contribution of BCAA to lipid biosynthesis and has not been explored beyond this.
In this study, we isolated and characterized several mutants of Chlamydomonas impaired in the branched-chain ketoacid dehydrogenase (BCKDH) complex. We show that during N starvation, BCAA degradation can influence TAG amount by 10% to 30%. In addition, we found that TAG remobilization following N resupply is also impaired in the mutants. This study thus provides genetic and biochemical evidence that BCAA catabolism contributes significantly to both biosynthesis and turnover of TAGs in Chlamydomonas, either by supplying reduced carbon precursors and ATP or through a signaling function.
RESULTS
The Pb8C12 Mutant Is Impaired in Both TAG Accumulation and Remobilization
To understand lipid synthesis and turnover processes in Chlamydomonas, a library of more than 4,500 mutants were screened for significant alterations in TAG contents under different N status (Cagnon et al., 2013). The mutant library was generated via random insertion of the APH8 gene encoding paromomycin resistance in the nuclear genome of Chlamydomonas (Cagnon et al., 2013). Among 80 mutants isolated, the Pb8C12 mutant accumulated less oil than the parental strain during mixotrophic N starvation (2 d) and was impaired in oil degradation following N resupply (1 d; Supplemental Fig. S1A). By contrast, no significant changes in the content and composition of polar lipids that are major components of membranes were found in this mutant (Supplemental Fig. S1B). However, a reduction in total fatty acids was observed in the mutant after N starvation (Supplemental Fig. S1C), which paralleled changes in TAG amounts, suggesting that the reduction in TAG resulted from a defect in the de novo fatty acid synthesis rather than from membrane lipid remodeling.
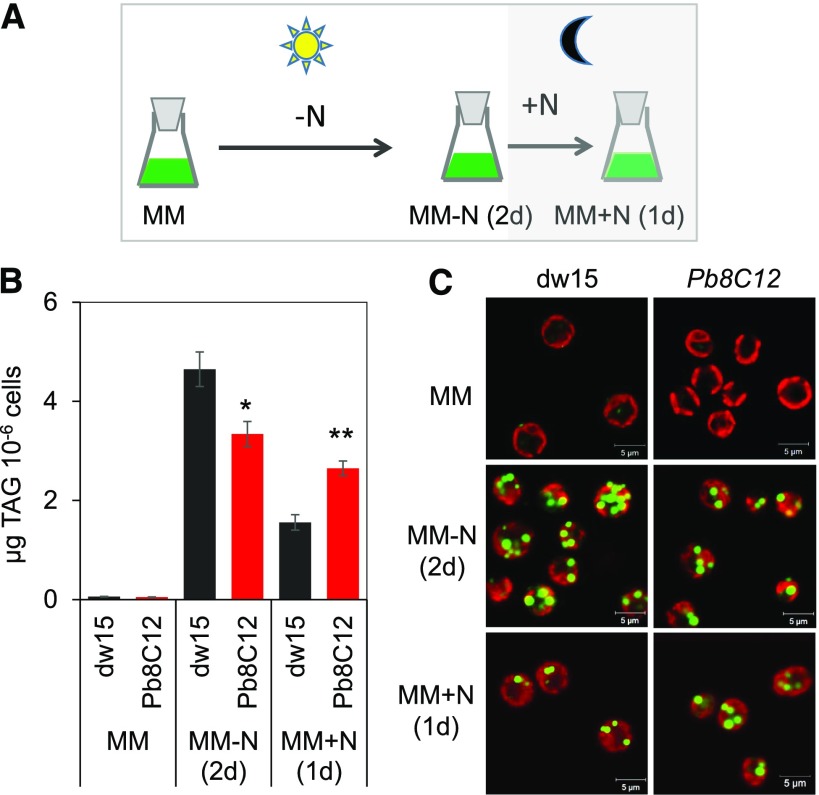
The initial screening was carried out in mixotrophic conditions (Cagnon et al., 2013; Supplemental Fig. S1), and here we show that under photoautotrophic cultivation, a similar conclusion can be drawn (Fig. 1, A and B). The Pb8C12 mutant accumulated 30% less oil than the wild type during N starvation (2 d), and it was also impaired in oil degradation following N resupply (i.e. ∼80% oil remaining 1 d after N resupply compared with only ∼30% remaining in the wild type when cells were kept in the dark; Fig. 1B). The delay in oil remobilization during recovery was further validated by imaging of the lipid droplets using confocal microscopy following cell staining with BODIPY (Fig. 1C). Moreover, when cells were kept in the light upon N resupply (i.e. during the remobilization phase), the mutant degraded its oil reserves as well as the wild type (Supplemental Fig. S2).
Figure 1.
Isolation of the Pb8C12 mutant in a genetic screen for TAG homeostasis. A, Cultivation conditions used for the genetic screen. B, TAG quantification. C, Lipid droplet imaging. Cells were cultivated until midlog phase photoautotrophically in the air with a supply of 2% CO2, then starved for N for 2 d, followed by N resupply. Cells were harvested at each stage for analysis of TAGs as well as for imaging. Data are means of four biological replicates, and error bars represent sd. Asterisks indicate significant differences from the wild-type strain dw15 using paired-sample Student’s t test (*, P ≤ 0.05 and **, P ≤ 0.01). Lipid droplets were stained with BODIPY. Pseudocolors were used: chlorophyll in red and lipid droplet in green. MM, Minimal medium.
Growth of the mutant cells was not affected in either mixotrophic or heterotrophic cultures (Supplemental Fig. S3, A and B). Intriguingly, in photoautotrophic conditions, the mutant reached a higher density at the stationery phase than its parental strain dw15, and we subsequently demonstrated that genetic complementation recovered the growth phenotype to wild-type levels (see later results on the generation of complemented lines; Supplemental Fig. S3C).
The Pb8C12 Mutant Contains an Insertion of the APH8 Gene in the Locus Encoding the E1α Subunit of the BCKDH Complex
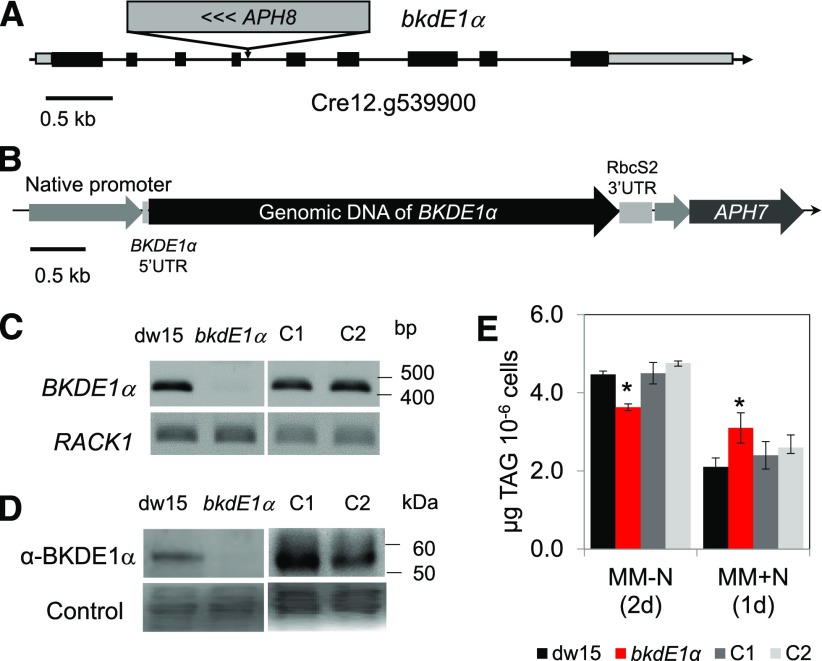
To understand the underlying genetic lesion in the mutant, the APH8 insertion site was identified by restriction enzyme site-directed amplification (RESDA)-PCR (González-Ballester et al., 2005; Kong and Li-Beisson, 2018). A primer specific to the APH8 gene, together with other degenerate primers, was used for PCR, which amplified a 705-bp fragment. DNA sequencing of this fragment identified the presence of a 421-bp DNA sequence from APH8 and a 284-bp DNA sequence from the bordering region. BLAST searches, using this flanking DNA sequence (284 bp) as bait and carried out within the genome of Chlamydomonas housed in Phytozome (V5.5), revealed that this sequence matched a region in the fourth intron of the locus Cre12.g539900. It was therefore assumed that the APH8 gene was inserted into Cre12.g539900, encoding a putative E1α subunit of the BCKDH protein complex (named hereafter BKDE1α; Fig. 2A). In plants as well as animals, the BCKDH complex is known to catalyze the second step in the degradation of BCAAs (i.e. the irreversible oxidative decarboxylation of α-keto acids to their respective acyl-CoAs; Binder, 2010).
Figure 2.
Mapping of the insertion site and genetic complementation. A, Locus of insertion in the Pb8C12 mutant. B, Gene construct for complementation. C, RT-PCR. D, Immunoblot analyses. E, TAG quantification. Cells were cultivated until midlog phase photoautotrophically in the air with a supply of 2% CO2. Cells were harvested for RT-PCR as well as for immunoblot analysis. Data are means of four biological replicates, and error bars represent sd. Asterisks indicate significant differences from the wild-type strain by paired-sample Student’s t test (*, P ≤ 0.05). For immunoblot, samples were loaded at equal total protein amounts and stained by Ponceau Red. RACK1, Receptor of activated protein C kinase1; UTR, untranslated region. C1 and C2 represent two independent complemented lines.
The knockout of this locus in the mutant Pb8C12 was confirmed by semiquantitative reverse transcription (RT)-PCR (Fig. 2C) as well as by immunoblot analysis using antibodies raised against the BKDE1α subunit (Fig. 2D). Although unspecific reactions were detected with the antibodies, the occurrence of a band of ∼55 kD, corresponding to the estimated molecular mass for BKDE1α protein (without the transit peptide) only in the wild type but not in the mutant, confirmed the absence of BKDE1α protein in the mutant (Fig. 2D). From here on, we renamed the Pb8C12 mutant bkdE1α.
The bkdE1α Mutant Is Complemented by Nuclear Expression of BKDE1α
The bkdE1α mutant was complemented using the genomic DNA of BKDE1α (5,567 bp) including a 1,028-bp promoter region, 5′ UTR (119 bp), and the coding region (Fig. 2B). After screening more than 100 hygromycin-resistant clones, two independent lines displayed recovered transcription of the BKDE1α gene (Fig. 2C), produced BKDE1α protein (Fig. 2D), and contained similar amounts of oil to the parental strain dw15 both during N starvation and upon N resupply (Fig. 2E).
Metabolomics Analysis Points to a Defect in BCAA Catabolism in bkdE1α
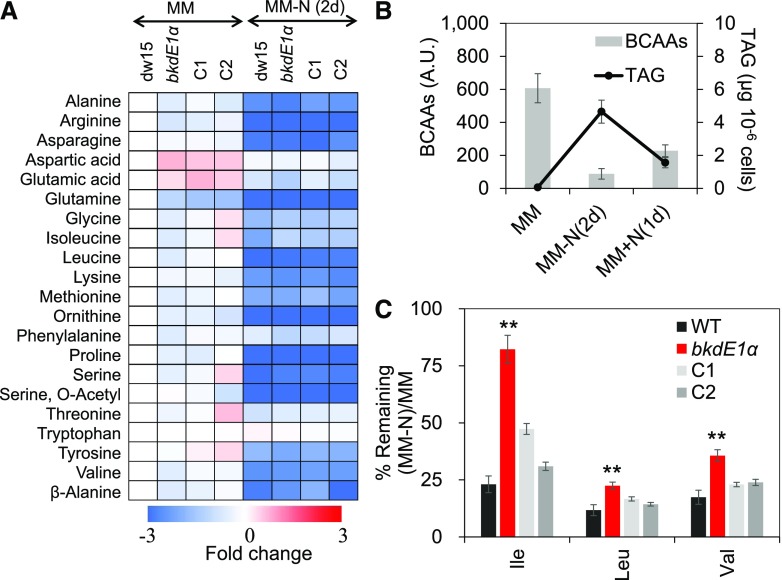
Mutants of the BCAA catabolic pathway often showed difference in free AA content (Peng et al., 2015). To test if this is also the case in the bkdE1a mutant, we analyzed free AAs in the mutant, its parental strain dw15, and two independent complemented lines (C1 and C2). We observed that in wild-type cells of Chlamydomonas, BCAAs represented around 20% of total free AAs (Supplemental Fig. S4) and all free AAs reduced in response to N depletion in all strains (Fig. 3A; Supplemental Fig. S5). The static level of free BCAAs correlated negatively with cellular TAG amount under varying N status (i.e. when TAG content is higher, free AAs are lower and vice versa; Fig. 3B), further suggesting that BCAAs could provide substrates for TAG synthesis.
Figure 3.
BCAA remobilization is impaired in the bkdE1a mutant. A, Free AA analyses by gas chromatography (GC)-mass spectrometry. B, BCAAs are in inverse relation to TAG content. C, Percentage of BCAAs remaining upon N starvation. Cells were cultivated until midlog phase photoautotrophically in the air with a supply of 2% CO2. Data are means of four biological replicates, and error bars represent sd. Asterisks indicate significant differences from control strains by paired-sample Student’s t test (**, P ≤ 0.01). A.U., Arbitrary units; WT, wild type.
Interestingly, the bkdE1α mutant made 20% to 30% less BCAAs during optimal growth, and the level of BCAAs reached close to that in the parental strain as well as complemented lines upon N depletion (Supplemental Fig. S5). When the capacity to remobilize BCAAs upon N starvation was calculated as a percentage of the remaining BCAA, we could see clearly that 1 d after N starvation, the bkdE1α mutant kept a significantly higher proportion of its BCAAs compared with the control strains (parental strain and complemented lines). This suggests a defect in the remobilization of BCAAs in the mutant (Fig. 3C). Taken together, these data confirm that BCKDH is involved in BCAA catabolism in Chlamydomonas. It is worth noting here that despite changes in BCAA catabolism, no significant change in protein content was detected (Supplemental Fig. S6).
Comparative RNA Sequencing Analysis Reveals the Activation of the BCAA Catabolic Pathway during N Starvation and upon N Resupply
In land plants and animals, the breakdown of BCAAs is known to occur in the mitochondria (Taylor et al., 2004; Angelovici et al., 2013). Major steps and proteins involved in the degradation of BCAAs have recently been described in the model plant Arabidopsis (Arabidopsis thaliana; Peng et al., 2015) as well as in tomato (Solanum lycopersicum; Kochevenko and Fernie, 2011; Kochevenko et al., 2012). Homology search of proteins similar to the known Arabidopsis ones have identified similar sets of proteins for BCAA degradation in Chlamydomonas (Table 1). Compared with Arabidopsis, fewer proteins are present. For example, only two genes encoding BCKDH E1 subunits are present, whereas Arabidopsis possesses four such genes. Except for the five putative branched-chain aminotransferase (BCATs), most other components of the pathway are predicted mitochondrial based on PredAlgo (Tardif et al., 2012) as well as TargetP (Emanuelsson et al., 2000). BCATs are predicted to be located at either chloroplast, mitochondria, or other, consistent with a dual role of these enzymes acting either at the last step of AA synthesis or at the first step of AA degradation (Binder et al., 2007; Angelovici et al., 2013).
Table 1. Putative proteins involved in BCAA catabolism in Arabidopsis and Chlamydomonas, their predicted subcellular localizations, and transcriptional responses.
PredAlgo was performed as explained by Tardif et al. (2012). C, Chloroplast; Log2FC, log2 fold change; M, mitochondria; NR, N recovery; O, other; –, not available. Night/Day data are from Zones et al. (2015).
| Annotation | Gene | TAIR ID | Phytozome ID | Log2FC (−N/+N) | Log2FC (NR/+N) | Night/Day | PredAlgo | TargetP |
|---|---|---|---|---|---|---|---|---|
| Branched-chain aminotransferase | BCAT1 | At1g10060 | Cre02.g081400 | 0.6 | −0.2 | 0.6 | C | C |
| BCAT2 | At1g10070 | Cre05.g245900 | −0.9 | −0.9 | 1.0 | C | M | |
| BCAT3 | At3g49680 | Cre10.g458050 | 1.2 | −0.9 | 0.2 | O | O | |
| BCAT4 | At3g19710 | Cre13.g576400 | −0.9 | 0.0 | 1.8 | C | C | |
| BCAT5 | At5g65780 | Cre02.g110700 | −1.7 | −1.8 | 0.9 | O | O | |
| BCAT6 | At1g50110 | – | – | – | – | – | – | |
| BCAT7 | At1g50090 | – | – | – | – | – | – | |
| α-Subunit of branched-chain ketoacid dehydrogenase E1 | BCKDH E1A1 | At1g21400 | – | – | – | – | – | – |
| BCKDH E1A2 (BKDE1α) | At5g09300 | Cre12.g539900 | 3.3 | 4.3 | 4.7 | M | M | |
| β-Subunit of branched-chain ketoacid dehydrogenase E1 | BCKDH E1B1 | At1g55510 | Cre06.g311050 | 3.4 | 3.5 | 9.0 | M | M |
| BCKDH E1B2 | At3g13450 | – | – | – | – | – | – | |
| Branched-chain ketoacid dehydrogenase E2 | BCKDH E2 | At3g06850 | Cre04.g228350 | 1.6 | 2.0 | 8.8 | M | M |
| Branched-chain ketoacid dehydrogenase E3 | BCKDH E3 mtLPD1 | At1g48030 | Cre18.g749847 | – | – | 0.8 | M | M |
| BCKDH E3 mtLPD2 | At3g17240 | – | – | – | – | – | – | |
| Isovaleryl-CoA dehydrogenase | IVD1 | At3g45300 | Cre06.g296400 | 2.1 | 1.9 | 7.8 | M | M |
| α-Subunit of 3-methylcrotonyl-CoA carboxylase | MCCA1 | At1g03090 | Cre06.g278098 | 2.3 | 2.9 | 12.7 | O | M |
| β-Subunit of 3-methylcrotonyl-CoA carboxylase | MCCB1 | At4g34030 | Cre03.g181200 | 2.3 | 2.7 | 4.6 | M | C |
| Hydroxymethylglutaryl-CoA lyase | HML1 | At2g26800 | Cre12.g485550 | – | −0.8 | 1.1 | M | M |
To quantify changes in the expression of genes of BCAA catabolism in response to N starvation and upon N resupply, we performed a comparative RNA sequencing (RNA-seq) experiment in the parental strain dw15. Total RNAs were extracted from dw15 cells harvested during normal growth, then 1 d after N starvation, and 1 d after N resupply. For each of the four biological replicates (i.e. cells harvested from independent shake-flask cultures), we mapped 22.4 to 30.5 million reads to the Chlamydomonas genome. The raw data were subjected to principal component analysis (Supplemental Fig. S7), which indicates a very good overall quality for the data obtained by RNA-seq. The DESeq2 package (Love et al., 2014) was used for the analysis of differential expression of the genes. Only genes with adjusted P ≤ 0.05 and an absolute log2FC ≥ 1 were kept for further analysis. Over 19,000 transcripts were detected and annotated. Mapping and gene annotation revealed that N starvation or resupply massively impacts genome transcription and that many metabolic pathways are affected (Supplemental Table S1, A and B). Since comparative transcriptome analyses of cells before and after N starvation have been previously published (Miller et al., 2010; Schmollinger et al., 2014; Tsai et al., 2014, 2018), we focused our analyses here on the changes in gene expression levels for genes of BCAA catabolism.
Interestingly, the BKDE1α gene was up-regulated (log2FC > 3) in response to N starvation as well as during the oil remobilization phase (i.e. upon N resupply [log2FC > 3]; Table 1). RNA-seq analyses of all putative genes of the BCAA degradation pathway indicate that, except for the E3 subunit (encoded by Cre18.g749847) of BCKDH and the five putative BCATs, all the others showed a similar up-regulation to BKDE1α both under N starvation as well as upon N resupply (Table 1). Most of them have also been found to be up-regulated in the night compared with the day in a diurnal cycle (Zones et al., 2015). The collective transcriptional response of the BCAA catabolic genes points to a common function of this catabolic pathway under nutrient stress, in the dark, or during carbon starvation, supporting the buffering role of BCAAs in supplying an alternative energy source when carbon or N levels are low.
Mutants Defective in Other Components of the BCKDH Complex Were Also Impaired in TAG Homeostasis
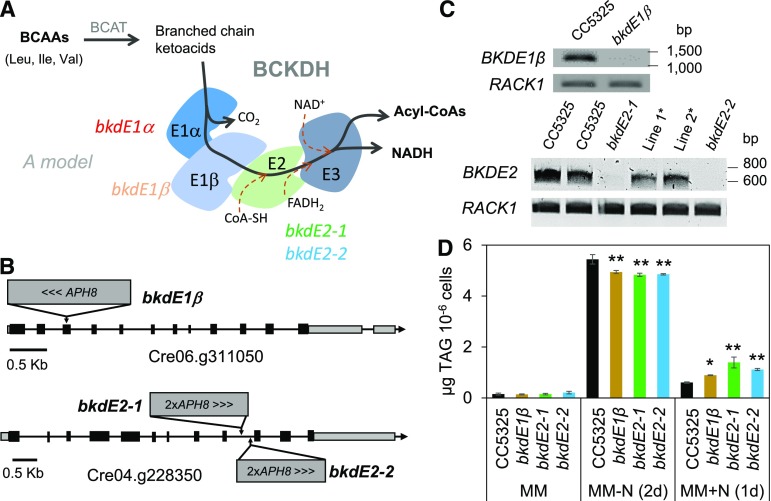
The BCKDH complex consists of three catalytic components: a heterotrimeric branched-chain α(β)-ketoacid dehydrogenase (E1; EC1.2.4.4), a dihydrolipoyl transacylase (E2; EC2.3.1.168), and a dihydrolipoamide dehydrogenase (E3; EC1.8.1.4; Fig. 4A). To further validate the link between the BCKDH complex and TAG homeostasis, we identified insertional mutants for E1β and E2 subunits from the Chlamydomonas library (CLIP [https://www.chlamylibrary.org/]; Li et al., 2016), such as LMJ.RY0402.153671-1 for the BKDE1β subunit and LMJ.RY0402.045578 and LMJ.RY0402.192581 for the BKDE2 subunit (Fig. 4B). The insertional event in each mutant was confirmed by PCR following the protocol given by the CLIP homepage (Supplemental Fig. S8), then the knockout of the corresponding gene was confirmed by RT-PCR analyses (Fig. 4C). These mutants were thus named as bkdE1β for LMJ.RY0402.153671-1, bkdE2-1 for LMJ.RY0402.045578, and bkdE2-2 for LMJ.RY0402.192581. TAG quantification revealed that all three mutants made ∼10% less oil than their parental strain during N starvation and showed a defect in oil remobilization following N resupply (Fig. 4D). The reduction in oil content (∼10%) is smaller compared with what was observed in the bkdE1α mutant (∼30%; Fig. 1). Various factors could explain such a difference, including the difference in the parental strain or in the contribution of these different subunits to activity of the BCKDH complex. Nevertheless, these data collectively point to a contribution of the BCKDH complex in TAG homeostasis in Chlamydomonas.
Figure 4.
Mutants deficient in other components of the BCKDH complex are also impaired in TAG homeostasis. A, Model for the BCKDH protein complex and putative reactions associated with it. B, The site of insertion(s) of APH8 in each individual genome. C, RT-PCR. D, TAG quantification. Cells were cultivated until midlog phase photoautotrophically in the air with a supply of 2% CO2. Data are means of four biological replicates, and error bars represent sd. Asterisks indicate significant differences from control strains by paired-sample Student’s t test (*, P ≤ 0.05 and **, P ≤ 0.01). Note that in C, Line 1* and Line 2* refer to RT-PCR analyses on two other independent isolates (ordered from the CLIP site: LMJ.RY0402.119905_1, and LMJ.RY0402.081600_1), which are proven not to be knockouts.
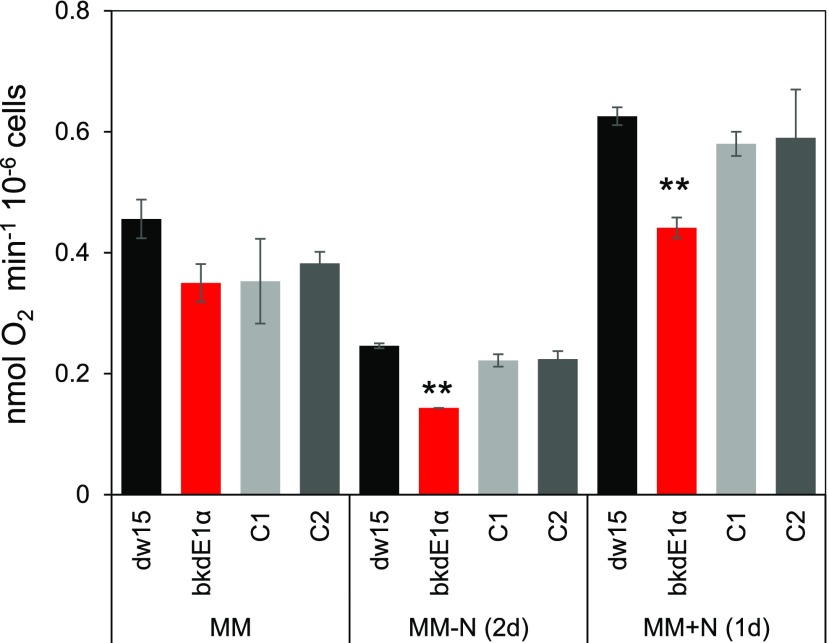
Mitochondrial Respiration Is Reduced in Mutants of the BCKDH Complex
BCAAs can serve as substrates for respiration, and to evaluate the impact on mitochondrial respiration, the rate of oxygen consumption in the dark was measured. The dark oxygen consumption rate was 20% to 35% lower in the bkdE1α mutant than in the control strains (dw15 and the two complemented lines, C1 and C2) before and after N starvation as well as upon N resupply (Fig. 5). A similar defect in mitochondrial respiration was also observed in the other three mutants defected in the distinct subunits of the BCKDH complex (i.e. BKDE1β and BKDE2; Supplemental Fig. S9). These data support the conclusion that a defect in BCAA catabolism affects mitochondrial respiration, providing evidence that BCAAs act as respiratory substrates.
Figure 5.
Mitochondrial respiration analyses of the bkdE1α mutant. Cells were cultivated until midlog phase photoautotrophically in the air with a supply of 2% CO2, then starved for N. Data are means of four biological replicates, and error bars represent sd. Asterisks indicate significant differences from control strains by paired-sample Student’s t test (**, P ≤ 0.01).
DISCUSSION
Due to the promise microalgae have as a next-generation platform for bioproducts, various ways to increase energy density in algal biomass have been explored intensively in the past 10 years. Among which, N starvation is one of the most potent triggers for initiating TAG accumulation; therefore, N starvation response has been investigated at multiple levels (Miller et al., 2010; Moellering and Benning, 2010; Siaut et al., 2011; Schmollinger et al., 2014). The outcome of this research is that in response to N starvation, significant metabolic changes occur: a massive accumulation of oil and starch, increased membrane lipid remodeling, a decrease in photosynthesis and photosynthetic pigments, and an increased rate of protein degradation and autophagy (Miller et al., 2010; Siaut et al., 2011; Davey et al., 2014; Schmollinger et al., 2014; Couso et al., 2018). While the effect of some pathways (notably starch) on oil accumulation has been studied, the interaction between AA metabolism and TAG synthesis remains less clear.
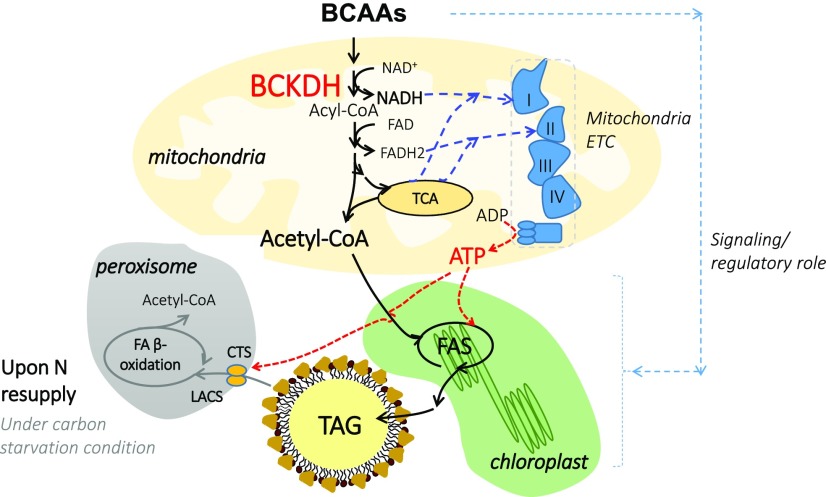
In this study, we demonstrate that BCAAs contribute significantly to TAG homeostasis. We further show that through its close interaction with the electron transport chain in mitochondria, BCAA catabolism also interacts with lipid degradation through the contribution of ATP for the initial steps of lipid catabolism. Thus, several potential metabolic interactions between BCAA catabolism and fatty acid synthesis and lipid degradation are drawn, and we propose that BCAA degradation provides not only carbon sources but also ATP for de novo fatty acid synthesis (Fig. 6). In addition to a metabolic connection, the relationship between BCAAs and TAG could result from BCAA-dependent signaling (Binder, 2010).
Figure 6.
A model explains the interaction between BCAA catabolism and TAG homeostasis in Chlamydomonas. Blue lines indicate potential flow of reducing equivalents; red lines indicate flow of ATP and black lines indicate carbon flow. When N starved, the free AA pool in cells is depleted. The degradation of some of these free AAs, namely BCAAs, produces acetyl-CoA as well as NADH, which is fed into the mitochondrial respiratory chain. The production of acetyl-CoA and ATP from BCAA catabolism contributes to TAG synthesis during N starvation. In addition, upon N resupply in the dark, the production of ATP through the BCAA catabolic pathway becomes essential to initiate the β-oxidation of fatty acids, as its earlier steps require two molecules of ATP (first, the transport of free fatty acids into peroxisomes is an ATP-requiring reaction, catalyzed by the ABC transporter called comatose [CTS]; second, the activation of free fatty acids to their CoA esters is an ATP-dependent reaction, catalyzed by long-chain acyl-CoA synthetase [LACS]). Furthermore, BCAA could also play a regulatory role in governing lipid homeostasis. ETC, Electron transport chain; FA, fatty acid; FAS, fatty acid synthase; TCA, tricarboxylic acid cycle.
BCAA Degradation and Lipid Biosynthesis
The bkdE1α mutant made 30% less TAG than control strains during N starvation (Figs. 1 and 2), indicating that BCAA acts as a carbon or energy source for fatty acid synthesis. A connection between BCAA catabolism and lipid synthesis has been evidenced recently in diatoms. Ge et al. (2014) found that, in parallel to the onset of massive oil accumulation in the model diatom P. tricornutum, proteins of BCAA catabolic pathways showed greater than 2-fold up-regulation; moreover, silencing the gene encoding the methylcrotonyl-CoA carboxylase (MCC2) resulted in strains making 27% to 43% less neutral lipids. This study suggests that BCAAs could act as substrates for TAG biosynthesis during N starvation in diatoms. Moreover, the occurrence of a relationship between BCAA catabolism and lipid synthesis is not limited to algae and has also been observed in mammalian cell lines. For example, it was found that inhibition of BCAA degradation in mouse cell lines decreased oil content, and the authors further report that BCAA contributed up to 30% acetyl-CoA required for lipogenesis in adipocytes (Green et al., 2016). For this reason, levels of BCAAs in mammalian cells have been used as a marker for obesity (Newgard, 2012; Crown et al., 2015; Green et al., 2016). Taken together, these studies point out the existence of a route for carbon flux from BCAAs to TAG in organisms of diverse evolutionary origin.
Nevertheless, in the current literature, the metabolic interaction between BCAA catabolism and TAG accumulation has not been explored beyond the contribution of carbon skeletons such as acetyl-CoAs. In addition to acetyl-CoA esters, the reaction catalyzed by the BCKDH complex produces NADH, which is believed to be fed to the internal alternative NADH dehydrogenase of complex I of the mitochondrial respiratory chain (Schertl and Braun, 2014), thereby influencing ATP production. Therefore, besides being a source of reduced carbon precursors, BCAAs are also respiratory substrates and their degradation is tightly connected to the mitochondrial respiratory chain. The impairment in the mitochondrial respiration of the bkdE1a mutant as well as the other mutants of the BCKDH complex provides clear evidence for this in algae (Fig. 5).
Taken together, BCAA catabolism in mitochondria provides carbon precursors for lipid synthesis, and in parallel, it provides direct electron donors to the mitochondrial electron transport chain and by so doing generates ATP. Acetyl-CoAs are essential building blocks for fatty acid synthesis, and ATP generated through mitochondrial respiration is an important source to sustain lipid synthesis, especially during stress conditions when the ATP production by the chloroplast photosynthesis is likely repressed (Schmollinger et al., 2014). The use of ATP generated in mitochondria to fulfill chloroplast functions was originally observed in the Chlamydomonas fud50 mutant deficient in the chloroplast ATP synthase (Lemaire et al., 1988). Later on, the occurrence of ATP trafficking from mitochondria to chloroplast was inferred in the Chlamydomonas mutant deficient in the Proton Gradient Regulation Like1 (Dang et al., 2014) and also in diatoms (Bailleul et al., 2015).
Considering the relatively small amount of free BCAAs (to account for the differences in TAG reduction in the mutant), together with the observation of no difference in protein amount between the bkdE1α mutant and its parental strain, we hypothesize that the interaction between BCAA and lipid could also be related to the signaling function of BCAAs (Kimball and Jefferson, 2006; Binder et al., 2007). The impact on oil homeostasis possibly results from an alteration in cell signaling networks in the absence of a functional BCKDH complex. The contribution of one or the other mechanism underlying the relationship between BCAA and lipids is worth further investigations.
BCAA Degradation and Lipid Catabolism
In mammalian cells, since fatty acid β-oxidation occurs essentially in mitochondria (Eaton et al., 1996), the metabolic link between storage lipid degradation and mitochondrial activity has been well established (Kujala et al., 2016). Based on our analysis and also based on the literature, BCAA degradation generally occurs in mitochondria, whereas the major steps for β-oxidation of fatty acids occur in peroxisomes of Chlamydomonas (Kong et al., 2017, 2018a, 2018b). This raises an interesting question, namely, how a defect in BCAA catabolism can affect lipid degradation. The reduction in mitochondrial respiration observed in the bkdE1α mutant probably provides an explanation (Fig. 5). Thus, it seems reasonable to postulate that less ATP is produced in the BCKDH-deficient mutants upon N resupply in the dark. The initial steps of β-oxidation of fatty acids require two ATPs, both to fuel the long-chain acyl-CoA synthetase (Fulda et al., 2004; Kong et al., 2018b) as well as to energize fatty acid import into the peroxisomes via an ATP-binding cassette transporter, the plant homolog of which is known to transfer fatty acids from cytosol into peroxisomes (Footitt et al., 2002). We thus propose that when mitochondrial respiration is impaired in the bkdE1α mutant, less ATP is produced, consequently fatty acid activation and import into peroxisomes are reduced, thereby impacting TAG breakdown (Fig. 6).
The observation that a defect in oil remobilization was observed in cells kept in the dark (Fig. 1B) but not in the light (Supplemental Fig. S2) provides compelling evidence for the importance of an initial supply of ATP in kicking off fatty acid β-oxidation. Taken together, these data suggest that some ATP is needed for the initiation of TAG degradation, ATP being possibly supplied by photosynthesis in the light or by mitochondrial respiration in the dark, to which BCAA breakdown most likely contributes. Indeed, in plants and algae, TAG is known not to be a good respiratory substrate, and the peroxisomal fatty acid β-oxidation pathway does not directly produce ATP. ATP production from fatty acid breakdown requires cooperation with the glyoxylate cycle and mitochondrial respiration (Kong et al., 2018b). Therefore, ATP production from TAG degradation in Chlamydomonas requires an initial ATP supply, even if TAG degradation will eventually generate a large amount of ATP and become self-sustained.
Defects in the BCKDH Complex on AA Metabolism and Cell Physiology
The level of free AAs depends on tissue types, developmental stage, or environmental conditions. In this study, we showed that AAs degrade massively during N starvation when TAGs accumulate (Fig. 3). This is consistent with the up-regulation of most genes of the BCAA catabolic pathway during the N starvation response (Table 1). This also correlates with the increase in most enzymes of BCAA catabolism in a quantitative proteomics study of P. tricornutum during the N starvation response (Ge et al., 2014).
It is worth noting here that metabolic changes were not limited to BCAAs in the bkdE1α mutant, and the levels of a few other AAs were also altered (Supplemental Fig. S5). The perturbation in BCAA catabolism has been observed to perturb the levels of other AAs in Arabidopsis (Peng et al., 2015) and in diatoms (Ge et al., 2014), which suggests a tight coordination in the synthesis and degradation of different types of AAs. Blocking BCAA catabolism in higher plants has resulted in the overaccumulation of BCAAs (Peng et al., 2015); however, this seems not to be the case in Chlamydomonas (this study). Many reasons could explain this discrepancy. It could be due to differences in carbon and energy metabolism in the green algae compared with plants, as has been shown before (Grossman et al., 2007; Liu and Benning, 2013). This could also be a result of the choice of sampling time point, and in this study, we analyzed only cells 2 d after N starvation (Fig. 3). Indeed, Leu overaccumulation was observed in MCC2-silenced diatom cells after 10 d of N starvation but not prior to this time point (Ge et al., 2014).
Intriguingly, we observed that the levels of BCAAs as well as several other AAs are low in the mutant as compared with the wild type (dw15) during optimal growth in the presence of N (Fig. 3; Supplemental Fig. S5). Actually, the amount of intracellular free BCAAs is the net result of de novo BCAA biosynthesis, protein degradation, BCAA catabolism, and their conversion/integration into other cellular components. A possible explanation could be that a defect in BCAA catabolism may somehow alter BCAA biosynthesis, possibly due to redox or ATP-dependent regulation in the chloroplast or to modifications in the BCAA-dependent intracellular signaling network.
During optimal growth, we observed that the bkdE1α mutant reached much higher cell density at the stationary phase than the parental strain dw15 as well as the two complemented strains, C1 and C2 (Supplemental Fig. S3). This contrasts with the lower cell density observed in strains silenced in the MCC2 gene in diatoms (Ge et al., 2014), reflecting the divergence in the evolutionary origin of these two groups of algae. Besides playing structural, storage, and respiratory roles, BCAAs are signaling molecules linking carbon to energy and to N metabolism (Araújo et al., 2010; Binder, 2010; Kochevenko et al., 2012; Hildebrandt et al., 2015), and the differences in growth behavior could be related to the use and uptake of N. Indeed, MCC2-silenced P tricornutum strains showed incomplete use of N from the medium. Further work is required to address this and other questions in the green alga Chlamydomonas.
MATERIALS AND METHODS
Strains, Mutant Isolation, and Culture Conditions
Chlamydomonas (Chlamydomonas reinhardtii) cell wall-less strain dw15.1 (nit1-305 cw15; mt+) was the parental strain to generate the mutant library from which the mutant Pb8C12 (bkdE1α) was derived (Cagnon et al., 2013). The strains were maintained on Tris-acetate phosphate (TAP) agar plates supplemented with 10 µg mL−1 paromomycin at 25°C under continuous illumination (about 30 μmol photons m−2 s−1). Liquid cultures were kept in incubation shakers (INFORS HT) at 25°C, 120 rpm shaking, with 75 μmol photons m−2 s−1 illumination. Cells were cultivated either mixotrophically (TAP) or photoautotrophically in MM with an addition of 2% (v/v) CO2 in the air, as specified in the text. To induce TAG accumulation, media without N (TAP-N or MM-N) were used. For TAG remobilization, N was added back to the cell culture while cells were kept in the dark. Therefore, a routine analysis of TAG homeostasis consists of the following: first, cells were cultivated in TAP or MM with the addition of 2% (v/v) CO2 in the air, transferred to TAP-N or MM-N (for 2 d), and then changed to MM that contains N but without acetate for another couple of days in the dark (Fig. 1A). Cell concentration was followed using a Multisizer 4 (MultisizerTM3 Coulter Counter; Beckman Coulter).
Isolation of Genomic DNA and RNA and cDNA Preparation
Genomic DNA or RNA was extracted from exponentially grown Chlamydomonas cells following the protocol of Tolleter et al. (2011) or Nguyen et al. (2013), respectively. Extracted total RNA was treated with TURBO DNase (Life Technologies) to remove any genomic DNA contamination. To obtain highly pure RNA, the total extracts were passed through a NucleoSpin RNA Clean-up column (Macherey-Nagel) following the manufacturer’s instructions. The first-strand complementary (DNA) was made using the SuperScript VILO cDNA Synthesis Kit (Thermo Fisher Scientific).
Identification of the APH8 Insertion Site in the Pb8C12 Mutant by RESDA-PCR
The insertion site of the APH8 cassette in the Pb8C12 mutant was identified by the RESDA-PCR method (González-Ballester et al., 2005) and also in Bioprotocols (Kong and Li-Beisson, 2018). Primers and PCR conditions were previously described (Kong et al., 2017). After BLAST searches of the Chlamydomonas genome (V5.5; Phytozome), the insertion cassette was found located in the fourth intron of the locus Cre12.g539900, which encodes the E1α subunit of a large enzymatic complex named BCKDH. The KOD Xtreme Hot Start DNA Polymerase (Novagen) was used for all PCR amplifications unless otherwise stated. All primer sequences used in this study are provided in Supplemental Table S2.
Isolation and Validation of Mutants from CLIP
Insertional mutant lines LMJ.RY0402.153671-1 (bkdE1β), LMJ.RY0402.045578 (bkdE2-1), LMJ.RY0402.192581 (bkdE2-2), and their parental line CC5325 were ordered from CLIP (https://www.chlamylibrary.org/; Li et al., 2016). These strains were streaked on agar plates containing paromomycin (10 µg mL−1) to obtain a single colony. Genomic DNA as well as RNA were extracted from cell cultures inoculated from a single colony. To validate a predicted insertional event, the following primer pairs were used: Control locus-F/Control locus-R (for control PCR), LMJ-gBKDE1β-F1/LMJ-gBKDE1β-R1 (for bkdE1β), LMJ-gBKDE2-F1/LMJ-gBKDE2-R1 (for bkdE2-1), and LMJ-gBKDE2-F2/LMJ-gBKDE2-R2 (for bkdE2-2).
RT-PCR
The expression levels of the different BCKDH subunits (BKDE1α, BKDE1β, and BKDE2) were evaluated by semiquantitative RT-PCR. RACK1 (Cre06.g278222) was used as a housekeeping gene. Primer pairs used for RT-PCR analyses were CBLP-F/CBLP-R (for RACK1), BCKDH-F/BCKDH-R (for BKDE1α), BKDE1β-F/BKDE1β-R (for BKDE1β), and BKDE2-F/BKDE2-R (for BKDE2).
Cloning the Full-Length BKDE1α and Genetic Complementation of the bkdE1α Mutant
The BKDE1α genomic DNA (Cre12.g539900) and its native promoter region (1,028 bp upstream of the 5′ UTR) together with the 3′ UTR of RbcS2 were cloned into the PAP22 vector (which was obtained by inserting a hygromycin gene to the blunt end of the PCR-blunt II-TOPO vector). Due to the presence of some complicated secondary structures around this region, and also due to their high GC content (67%), we cloned the full-length sequence by two overlapping fragments: (1) the first fragment, containing the promoter region to the end of the fourth exon, was amplified using the primer pair CrBCKDH-XbaI-FP3/CrBCKDH-R6; (2) the second fragment was cloned from the beginning of the fourth exon to the stop codon using the primer pair CrBCKDH-F6/CrBCKDH-NdeI-R2. These two fragments were then ligated into a single fragment by PCR. Briefly, the above two fragments were purified and mixed in an equal molar ratio (500 ng each) to serve as a template for PCR amplifications. PCR was carried out following the KOD Xtreme Hot Start DNA Polymerase (Novagen) protocol without primers (94°C for 2 min, then 11× 98°C for 10 s and 74°C for 3 min 10 s, and finally one cycle at 74°C for 5 min). Then, 2 µL of the above reaction was used as a template to allow PCR amplification using primers CrBCKDH-XbaI-FP3 and CrBCKDH-NdeI-R2. The entire gene sequence was then confirmed by DNA sequencing (at GATC). Finally, the full-length sequence (5,349 bp), together with the 3′ UTR of RbcS2 (from pChalmy_4 vector [Life Technologies] using RbcS3Ter-NdeI and RbcS2-NotI primers), was ligated to the PAP22 vector (digested by XbaI and NotI). The linearized vector cut with XmaI was electroporated to the bkdE1α mutant cells, and antibiotic-resistant clones were selected on agar plates containing 17 µg mL−1 hygromycin.
The Use of Flow Cytometry to Screen for Complemented Lines
Cellular oil content of independent transformants was first screened using flow cytometry after cells were stained with BODIPY 505/515 (D-3921; 4,4-difluoro-1,3,5,7-tetramethyl-4-bora-3a,4a-diaza-s-indacene [Thermo Fisher Scientific]; Mou et al., 2012). BODIPY was added to cell cultures at a final concentration of 0.25 µg mL−1 (from a stock solution of 50 μg mL−1 BODIPY solubilized in dimethyl sulfoxide) and incubated in the dark for 5 min at room temperature before analyses. Neutral lipids stained with BODIPY show an emission peak at 521 nm when excited by a 488-nm laser line. Cultivation conditions for the 96-well plate cultures used were as detailed by Cagnon et al. (2013). Clones showing recovered oil content similar to the wild-type strain based on flow cytometry analyses were then confirmed by TAG quantification using thin layer chromatography (TLC).
Lipid Extraction and Quantification
Total lipids were extracted from exponentially grown Chlamydomonas cells using a modified method of Bligh and Dyer (1959) and detailed in Siaut et al. (2011). TAG amount as well as amounts of other polar lipids were quantified using high-performance TLC (CAMAG). Briefly, a given amount of lipid extracts was deposited on a 10- × 20-cm silica gel 60 F254 TLC plate (Merck) using an ATS5 automatic TLC sampler. The TLC plate was developed either in a mixture of hexane:diethyl ether:acetic acid (17:3:0.2, v/v/v) for neutral lipid separation or in a mixture of acetone:toluene:water (91:30:8, v/v/v) for polar lipid separation. After development, the plates were air dried, and lipids were revealed after dipping in a mixture of 20 g of CuSO4 and 80 mL of H3PO4 and then heated at 170°C for 20 min. The lipid standards used were triheptadecanoin (C17:0 TAG; Sigma-Aldrich), monogalactosyl distearoylglyceride (Larodan Fine Chemicals), digalactosyl distearoylglyceride (Larodan), 1,2-dipalmitoyl-sn-glycerol-3-phospho-(1′-rac-glycerol; Avanti), and 1,2-dipalmitoyl-sn-glycerol-3-phosphoethanolamine (Avanti).
Fatty Acid Compositional Analysis by GC
Fatty acid composition in a lipid extract, or a whole cell, can be analyzed by GC after being converted to the more volatile fatty acid methyl esters (FAMEs). In this study, we routinely analyzed fatty acid composition via direct transmethylation of cell pellets. Briefly, 1 mL of 5% (v/v) sulfuric acid in methanol and 10 μL of 1% butylated hydroxytoluene were added to a glass tube containing 20 million freshly harvested cells. C17:0 TAG (10 μg) was added as an internal standard for quantification and as a control for the efficiency of the transesterification reaction. The mixture was vortexed and heated for 90 min at 85°C. After cooling down to room temperature, 1.5 mL of 0.9% (w/v) NaCl was added to the reaction, and lipophilic products were extracted three times with hexane. To allow easier phase separation, the mixture was then centrifuged at 3,000 g for 2 min. The upper hexane phase containing FAMEs was transferred to a clean tube and evaporated to dryness under a gentle N stream. The FAMEs were then dissolved in 200 μL of hexane, transferred to a GC vial, and analyzed by a GC-flame ionization detection apparatus (7890A gas chromatograph and 5975C mass spectrometer; Agilent Technologies) using a polar TR-WAX column (30 m × 0.25 mm × 0.5 μm). The GC conditions were as follows: split ratio of 1:20; injector and flame ionization detector temperature of 240°C; and oven temperature program of 50°C for 2 min, then increasing at 15°C min−1 to 150°C, and then increasing again at 6°C min−1 to 240°C, and holding at this temperature for 4 min. The flow rate of the carrier gas (H2) was 1 mL min−1.
Lipid Droplet Imaging
To observe lipid droplets, Chlamydomonas cells were first enriched via a gentle centrifugation (850 g, 3 min) and then resuspended in a small amount (100–200 µL) of fresh medium with 0.25% glutaraldehyde. Cells were stained with BODIPY (to a final concentration of 0.25 µg mL−1, from a stock of 50 µg mL−1 in dimethyl sulfoxide) in the dark for 5 min. Cells were then excited with a laser line at 488 nm, and emission was collected between 500 and 545 nm for BODIPY and between 650 and 730 nm for chlorophyll autofluorescence. All were carried out under a 63× oil-immersion objective in a confocal laser scanning microscope (TCS SP2; Leica). Pseudocolors were applied using the ZEN (Carl Zeiss) software.
Protein Extraction and Quantification
To examine whether the wild type and the bkdA1 mutant differ significantly in bulk protein, total proteins were extracted from exponentially grown cells and from cells subjected to N starvation as well as N resupply. Twenty million cells were harvested by centrifugation for 3 min at 850g and resuspended in 1 mL of lysis buffer (50 mm HEPES-KOH, pH 7.5, 1 mm EDTA, 5 mm dithiothreitol, 10% [v/v] glycerol, and 0.1× protease inhibitor cocktail; Sigma P9599). Cells were sonicated on ice for 42 s with an alternating cycle of 7-s pulse/3-s pause. Lysates were transferred into 1.5-mL Eppendorf tubes and centrifuged at 13,000 g for 10 min at 4°C. The supernatant (300 µL) was transferred to a new tube, to which 1,200 μL of acetone (at −20°C) was added. The mixture was vortexed and incubated for more than 1 h at −20°C. The sample was centrifuged again at maximum speed for 15 min at 4°C; the supernatant was decanted and air dried for 5 min and resolubilized in 200 μL of 0.2% SDS to use for protein quantification following the protocol of the Pierce BCA protein assay kit (Thermo Fisher Scientific).
Antibody Generation and Immunoblot Analysis
Polyclonal antibodies against the synthetic peptide (AA380-394; Cys-SGGLLTEPAVGAVGK) were immunized in two rabbits (ProteoGenix; SAS). For immunoblot analysis, total protein extracts (10 µg) were separated on a 10% Bis-Tris gel using MES running buffer, transferred to a BioTrace NT nitrocellulose membrane (Sigma-Aldrich), and immunoblotted with specific primary polyclonal antibodies (1:500) from rabbit at room temperature for 2 h. This was followed by incubation with a secondary anti-rabbit antibody (Invitrogen) for 1 h. Immobilon Western Chemiluminescent HRP Substrate (EMD Millipore) was used for the detection, and images were recorded using a G:BOX Chemi XL (Syngene).
AA Analyses by GC-Mass Spectrometry
Chlamydomonas cells (60 million) were harvested quickly by centrifugation at 13,000g for 10 s at 4°C and then immediately frozen in liquid N. Metabolite extraction and derivatization were as described previously (Lisec et al., 2006) and then quantified using an Agilent 7683 series autosample (Agilent Technologies) coupled to an Agilent 6890 gas chromatograph-Leco Pegasus two time-of-flight mass spectrometer (Leco). The chromatogram parameters and analytical part were exactly as has been reported previously (Cuadros-Inostroza et al., 2009; Kong et al., 2018a).
Respiration Analysis Using a Clark Electrode
The mitochondrial respiration rate was determined in the dark using a Clark-type oxygen electrode that consists of a cathode (platinum electrode) and an anode (Ag/AgCl electrode) linked by a concentrated KCl solution (Hansatech Instruments). The oxygen consumption of the cathode is stoichiometrically related to the electrical current, so a change in the oxygen concentration by respiration can be easily determined by measuring the electrical current produced. Before measurement, the instrument was first calibrated to 0 by adding sodium dithionite, which can consume all the molecular oxygen present in the air-saturated water, and then the oxygen concentration value of air-saturated water was set as 1,000. Finally, the respiration rate of cell culture was calculated based on the data recorded on the PicoScope2202 recorder software (Pico Technology; Interworld Electronics).
RNA-Seq
RNA was extracted from dw15 cells harvested under three different N statuses (+N, −N, and N recovery; four replicates for each condition) using the TruSeq RNA Sample Preparation Kit (Illumina). Then a cDNA library was built from 1 µg of total RNA, and Illumina HiSeq 2500 sequencing was performed by the Biopuces and Sequencage platform at Illkirch, generating 27.6 to 38.1 million 50-nucleotide single-end reads for each replicate (Supplemental Table S1C). Reads were aligned using Bowtie2 software (Langmead and Salzberg, 2012) onto the Chlamydomonas genome assembly version V5.5, and high throughput sequencing count (Love et al., 2014) was used to identify reads uniquely mapped on the genome. Differentially expressed genes were classified in metabolic pathways and gene categories using the MapMan application software (https://mapman.gabipd.org/).
Statistical Analysis
Student’s t test was performed on biological replicates (i.e. cells harvested from independent cultures) using Excel software (2013 version).
Accession Numbers
All accession numbers used in the article are listed in Table 1.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Lipid analyses during mixotrophic N starvation.
Supplemental Figure S2. TAG remobilization is not affected in the bkdE1α mutant under light.
Supplemental Figure S3. Growth kinetics.
Supplemental Figure S4. Free AA composition in the parental strain dw15.
Supplemental Figure S5. Free AA amount before and after N starvation.
Supplemental Figure S6. Total protein content.
Supplemental Figure S7. Data quality analysis for RNA-seq.
Supplemental Figure S8. PCR validation of APH8 gene insertion in other mutants of the BCKDH complex.
Supplemental Figure S9. Mitochondrial respiration analysis of the bkdE1β and bkdE2 mutants.
Supplemental Table S1. RNA-seq analyses.
Supplemental Table S2. Primers used in this study.
Acknowledgments
We thank Pascaline Auroy, Stephanie Blangy, and Audrey Beyly for technical help.
Footnotes
This work was supported by an Amidex from Aix-Marseille University. Y.L. was supported by the China Scholarship Council (CSC) for a Ph.D. student stipend. I.T.R. and A.B. were supported by the Commissariat à l’Energie Atomique for an international Ph.D. studentship (Irtelis).The European Union Regional Developing Fund (ERDF), the Région Provence Alpes Côte d’Azur, the French Ministry of Research, and the Commissariat à l’Energie Atomique funded the HelioBiotec platform. The European Union also provided funding in the framework of the European Union 2020 TEAMING Project (SGA-CSA no. 664621 and no. 739582 under FPA no. 664620 to S.A. and A.R.F.).
Articles can be viewed without a subscription.
References
- Angelovici R, Lipka AE, Deason N, Gonzalez-Jorge S, Lin H, Cepela J, Buell R, Gore MA, Dellapenna D (2013) Genome-wide analysis of branched-chain amino acid levels in Arabidopsis seeds. Plant Cell 25: 4827–4843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo WL, Ishizaki K, Nunes-Nesi A, Larson TR, Tohge T, Krahnert I, Witt S, Obata T, Schauer N, Graham IA, et al. (2010) Identification of the 2-hydroxyglutarate and isovaleryl-CoA dehydrogenases as alternative electron donors linking lysine catabolism to the electron transport chain of Arabidopsis mitochondria. Plant Cell 22: 1549–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidan O, Brandis A, Rogachev I, Pick U (2015) Enhanced acetyl-CoA production is associated with increased triglyceride accumulation in the green alga Chlorella desiccata. J Exp Bot 66: 3725–3735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul B, Berne N, Murik O, Petroutsos D, Prihoda J, Tanaka A, Villanova V, Bligny R, Flori S, Falconet D, et al. (2015) Energetic coupling between plastids and mitochondria drives CO2 assimilation in diatoms. Nature 524: 366–369 [DOI] [PubMed] [Google Scholar]
- Binder S. (2010) Branched-chain amino acid metabolism in Arabidopsis thaliana. The Arabidopsis Book 8: e0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder S, Knill T, Schuster J (2007) Branched-chain amino acid metabolism in higher plants. Physiol Plant 129: 68–78 [Google Scholar]
- Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917 [DOI] [PubMed] [Google Scholar]
- Bourgis F, Kilaru A, Cao X, Ngando-Ebongue GF, Drira N, Ohlrogge JB, Arondel V (2011) Comparative transcriptome and metabolite analysis of oil palm and date palm mesocarp that differ dramatically in carbon partitioning. Proc Natl Acad Sci USA 108: 12527–12532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle NR, Page MD, Liu B, Blaby IK, Casero D, Kropat J, Cokus SJ, Hong-Hermesdorf A, Shaw J, Karpowicz SJ, et al. (2012) Three acyltransferases and nitrogen-responsive regulator are implicated in nitrogen starvation-induced triacylglycerol accumulation in Chlamydomonas. J Biol Chem 287: 15811–15825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnon C, Mirabella B, Nguyen HM, Beyly-Adriano A, Bouvet S, Cuiné S, Beisson F, Peltier G, Li-Beisson Y (2013) Development of a forward genetic screen to isolate oil mutants in the green microalga Chlamydomonas reinhardtii. Biotechnol Biofuels 6: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JE, Smith AG (2012) A look at diacylglycerol acyltransferases (DGATs) in algae. J Biotechnol 162: 28–39 [DOI] [PubMed] [Google Scholar]
- Couso I, Pérez-Pérez ME, Martínez-Force E, Kim HS, He Y, Umen JG, Crespo JL (2018) Autophagic flux is required for the synthesis of triacylglycerols and ribosomal protein turnover in Chlamydomonas. J Exp Bot 69: 1355–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown SB, Marze N, Antoniewicz MR (2015) Catabolism of branched chain amino acids contributes significantly to synthesis of odd-chain and even-chain fatty acids in 3T3-L1 adipocytes. PLoS ONE 10: e0145850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadros-Inostroza A, Caldana C, Redestig H, Kusano M, Lisec J, Peña-Cortés H, Willmitzer L, Hannah MA (2009) TargetSearch: A Bioconductor package for the efficient preprocessing of GC-MS metabolite profiling data. BMC Bioinformatics 10: 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang KV, Plet J, Tolleter D, Jokel M, Cuiné S, Carrier P, Auroy P, Richaud P, Johnson X, Alric J, et al. (2014) Combined increases in mitochondrial cooperation and oxygen photoreduction compensate for deficiency in cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell 26: 3036–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MP, Horst I, Duong GH, Tomsett EV, Litvinenko ACP, Howe CJ, Smith AG (2014) Triacylglyceride production and autophagous responses in Chlamydomonas reinhardtii depend on resource allocation and carbon source. Eukaryot Cell 13: 392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton S, Bartlett K, Pourfarzam M (1996) Mammalian mitochondrial beta-oxidation. Biochem J 320: 345–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Fan J, Andre C, Xu C (2011) A chloroplast pathway for the de novo biosynthesis of triacylglycerol in Chlamydomonas reinhardtii. FEBS Lett 585: 1985–1991 [DOI] [PubMed] [Google Scholar]
- Fan J, Yan C, Andre C, Shanklin J, Schwender J, Xu C (2012) Oil accumulation is controlled by carbon precursor supply for fatty acid synthesis in Chlamydomonas reinhardtii. Plant Cell Physiol 53: 1380–1390 [DOI] [PubMed] [Google Scholar]
- Footitt S, Slocombe SP, Larner V, Kurup S, Wu Y, Larson T, Graham I, Baker A, Holdsworth M (2002) Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J 21: 2912–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda M, Schnurr J, Abbadi A, Heinz E, Browse J (2004) Peroxisomal acyl-CoA synthetase activity is essential for seedling development in Arabidopsis thaliana. Plant Cell 16: 394–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge F, Huang W, Chen Z, Zhang C, Xiong Q, Bowler C, Yang J, Xu J, Hu H (2014) Methylcrotonyl-CoA carboxylase regulates triacylglycerol accumulation in the model diatom Phaeodactylum tricornutum. Plant Cell 26: 1681–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Kolbe A, Tiessen A (2005) Redox regulation of carbon storage and partitioning in response to light and sugars. J Exp Bot 56: 1469–1479 [DOI] [PubMed] [Google Scholar]
- González-Ballester D, de Montaigu A, Galván A, Fernández E (2005) Restriction enzyme site-directed amplification PCR: A tool to identify regions flanking a marker DNA. Anal Biochem 340: 330–335 [DOI] [PubMed] [Google Scholar]
- Goodenough U, Blaby I, Casero D, Gallaher SD, Goodson C, Johnson S, Lee JH, Merchant SS, Pellegrini M, Roth R, et al. (2014) The path to triacylglyceride obesity in the sta6 strain of Chlamydomonas reinhardtii. Eukaryot Cell 13: 591–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson C, Roth R, Wang ZT, Goodenough U (2011) Structural correlates of cytoplasmic and chloroplast lipid body synthesis in Chlamydomonas reinhardtii and stimulation of lipid body production with acetate boost. Eukaryot Cell 10: 1592–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goold HD, Cuiné S, Légeret B, Liang Y, Brugière S, Auroy P, Javot H, Tardif M, Jones B, Beisson F, et al. (2016) Saturating light induces sustained accumulation of oil in plastidal lipid droplets in Chlamydomonas reinhardtii. Plant Physiol 171: 2406–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CR, Wallace M, Divakaruni AS, Phillips SA, Murphy AN, Ciaraldi TP, Metallo CM (2016) Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat Chem Biol 12: 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AR, Croft M, Gladyshev VN, Merchant SS, Posewitz MC, Prochnik S, Spalding MH (2007) Novel metabolism in Chlamydomonas through the lens of genomics. Curr Opin Plant Biol 10: 190–198 [DOI] [PubMed] [Google Scholar]
- Hildebrandt TM, Nunes Nesi A, Araújo WL, Braun HP (2015) Amino acid catabolism in plants. Mol Plant 8: 1563–1579 [DOI] [PubMed] [Google Scholar]
- Kajikawa M, Sawaragi Y, Shinkawa H, Yamano T, Ando A, Kato M, Hirono M, Sato N, Fukuzawa H (2015) Algal dual-specificity tyrosine phosphorylation-regulated kinase, triacylglycerol accumulation regulator1, regulates accumulation of triacylglycerol in nitrogen or sulfur deficiency. Plant Physiol 168: 752–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS (2006) Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr (Suppl) 136: 227S–231S [DOI] [PubMed] [Google Scholar]
- Kochevenko A, Fernie AR (2011) The genetic architecture of branched-chain amino acid accumulation in tomato fruits. J Exp Bot 62: 3895–3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochevenko A, Araújo WL, Maloney GS, Tieman DM, Do PT, Taylor MG, Klee HJ, Fernie AR (2012) Catabolism of branched chain amino acids supports respiration but not volatile synthesis in tomato fruits. Mol Plant 5: 366–375 [DOI] [PubMed] [Google Scholar]
- Kong F, Li-Beisson Y (2018) Identification of insertion site by RESDA-PCR in Chlamydomonas mutants generated by AphVIII random insertional mutagenesis. Bio Protoc 8: e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, Liang Y, Légeret B, Beyly-Adriano A, Blangy S, Haslam RP, Napier JA, Beisson F, Peltier G, Li-Beisson Y (2017) Chlamydomonas carries out fatty acid β-oxidation in ancestral peroxisomes using a bona fide acyl-CoA oxidase. Plant J 90: 358–371 [DOI] [PubMed] [Google Scholar]
- Kong F, Burlacot A, Liang Y, Légeret B, Alseekh S, Brotman Y, Fernie AR, Krieger-Liszkay A, Beisson F, Peltier G, et al. (2018a) Interorganelle communication: Peroxisomal MALATE DEHYDROGENASE2 connects lipid catabolism to photosynthesis through redox coupling in Chlamydomonas. Plant Cell 30: 1824–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, Romero IT, Warakanont J, Li-Beisson Y (2018b) Lipid catabolism in microalgae. New Phytol 218: 1340–1348 [DOI] [PubMed] [Google Scholar]
- Krishnan A, Kumaraswamy GK, Vinyard DJ, Gu H, Ananyev G, Posewitz MC, Dismukes GC (2015) Metabolic and photosynthetic consequences of blocking starch biosynthesis in the green alga Chlamydomonas reinhardtii sta6 mutant. Plant J 81: 947–960 [DOI] [PubMed] [Google Scholar]
- Kujala UM, Peltonen M, Laine MK, Kaprio J, Heinonen OJ, Sundvall J, Eriksson JG, Jula A, Sarna S, Kainulainen H (2016) Branched-chain amino acid levels are related with surrogates of disturbed lipid metabolism among older men. Front Med (Lausanne) 3: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire C, Wollman FA, Bennoun P (1988) Restoration of phototrophic growth in a mutant of Chlamydomonas reinhardtii in which the chloroplast atpB gene of the ATP synthase has a deletion: An example of mitochondria-dependent photosynthesis. Proc Natl Acad Sci USA 85: 1344–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Moellering ER, Liu B, Johnny C, Fedewa M, Sears BB, Kuo MH, Benning C (2012) A galactoglycerolipid lipase is required for triacylglycerol accumulation and survival following nitrogen deprivation in Chlamydomonas reinhardtii. Plant Cell 24: 4670–4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang R, Patena W, Gang SS, Blum SR, Ivanova N, Yue R, Robertson JM, Lefebvre PA, Fitz-Gibbon ST, et al. (2016) An indexed, mapped mutant library enables reverse genetics studies of biological processes in Chlamydomonas reinhardtii. Plant Cell 28: 367–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Han D, Hu G, Dauvillee D, Sommerfeld M, Ball S, Hu Q (2010) Chlamydomonas starchless mutant defective in ADP-glucose pyrophosphorylase hyper-accumulates triacylglycerol. Metab Eng 12: 387–391 [DOI] [PubMed] [Google Scholar]
- Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, DeBono A, Durrett TP, et al. (2013) Acyl-lipid metabolism. The Arabidopsis Book 11: e0161, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR (2006) Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc 1: 387–396 [DOI] [PubMed] [Google Scholar]
- Liu B, Benning C (2013) Lipid metabolism in microalgae distinguishes itself. Curr Opin Biotechnol 24: 300–309 [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelet L, Zaffagnini M, Morisse S, Sparla F, Pérez-Pérez ME, Francia F, Danon A, Marchand CH, Fermani S, Trost P, et al. (2013) Redox regulation of the Calvin-Benson cycle: Something old, something new. Front Plant Sci 4: 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R, Wu G, Deshpande RR, Vieler A, Gärtner K, Li X, Moellering ER, Zäuner S, Cornish AJ, Liu B, et al. (2010) Changes in transcript abundance in Chlamydomonas reinhardtii following nitrogen deprivation predict diversion of metabolism. Plant Physiol 154: 1737–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering ER, Benning C (2010) RNA interference silencing of a major lipid droplet protein affects lipid droplet size in Chlamydomonas reinhardtii. Eukaryot Cell 9: 97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou SL, Xu D, Ye NH, Zhang XW, Liang CW, Liang Q, Zheng Z, Zhuang ZM, Miao JL (2012) Rapid estimation of lipid content in an Antarctic ice alga (Chlamydomonas sp.) using the lipophilic fluorescent dye BODIPY505/515. J Appl Phycol 24: 1169–1176 [Google Scholar]
- Newgard CB. (2012) Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 15: 606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HM, Cuiné S, Beyly-Adriano A, Légeret B, Billon E, Auroy P, Beisson F, Peltier G, Li-Beisson Y (2013) The green microalga Chlamydomonas reinhardtii has a single ω-3 fatty acid desaturase that localizes to the chloroplast and impacts both plastidic and extraplastidic membrane lipids. Plant Physiol 163: 914–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J, Browse J (1995) Lipid biosynthesis. Plant Cell 7: 957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Uygun S, Shiu SH, Last RL (2015) The impact of the branched-chain ketoacid dehydrogenase complex on amino acid homeostasis in Arabidopsis. Plant Physiol 169: 1807–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan R, Kim BH, Cho DH, Ko SR, Oh HM, Kim HS (2013) Lipid droplet synthesis is limited by acetate availability in starchless mutant of Chlamydomonas reinhardtii. FEBS Lett 587: 370–377 [DOI] [PubMed] [Google Scholar]
- Schertl P, Braun HP (2014) Respiratory electron transfer pathways in plant mitochondria. Front Plant Sci 5: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmollinger S, Mühlhaus T, Boyle NR, Blaby IK, Casero D, Mettler T, Moseley JL, Kropat J, Sommer F, Strenkert D, et al. (2014) Nitrogen-sparing mechanisms in Chlamydomonas affect the transcriptome, the proteome, and photosynthetic metabolism. Plant Cell 26: 1410–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Raffelt M, Chochois V, Auroy P, Cuiné S, Billon E, Dauvillée D, Li-Beisson Y, Peltier G (2016) Hyper-accumulation of starch and oil in a Chlamydomonas mutant affected in a plant-specific DYRK kinase. Biotechnol Biofuels 9: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siaut M, Cuiné S, Cagnon C, Fessler B, Nguyen M, Carrier P, Beyly A, Beisson F, Triantaphylidès C, Li-Beisson Y, et al. (2011) Oil accumulation in the model green alga Chlamydomonas reinhardtii: Characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol 11: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif M, Atteia A, Specht M, Cogne G, Rolland N, Brugière S, Hippler M, Ferro M, Bruley C, Peltier G, et al. (2012) PredAlgo: A new subcellular localization prediction tool dedicated to green algae. Mol Biol Evol 29: 3625–3639 [DOI] [PubMed] [Google Scholar]
- Taylor NL, Heazlewood JL, Day DA, Millar AH (2004) Lipoic acid-dependent oxidative catabolism of alpha-keto acids in mitochondria provides evidence for branched-chain amino acid catabolism in Arabidopsis. Plant Physiol 134: 838–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolleter D, Ghysels B, Alric J, Petroutsos D, Tolstygina I, Krawietz D, Happe T, Auroy P, Adriano JM, Beyly A, et al. (2011) Control of hydrogen photoproduction by the proton gradient generated by cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell 23: 2619–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CH, Warakanont J, Takeuchi T, Sears BB, Moellering ER, Benning C (2014) The protein Compromised Hydrolysis of Triacylglycerols 7 (CHT7) acts as a repressor of cellular quiescence in Chlamydomonas. Proc Natl Acad Sci USA 111: 15833–15838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CH, Uygun S, Roston R, Shiu SH, Benning C (2018) Recovery from N deprivation is a transcriptionally and functionally distinct state in Chlamydomonas. Plant Physiol 176: 2007–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzica EI, Vieler A, Hong-Hermesdorf A, Page MD, Casero D, Gallaher SD, Kropat J, Pellegrini M, Benning C, Merchant SS (2013) Remodeling of membrane lipids in iron-starved Chlamydomonas. J Biol Chem 288: 30246–30258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZT, Ullrich N, Joo S, Waffenschmidt S, Goodenough U (2009) Algal lipid bodies: Stress induction, purification, and biochemical characterization in wild-type and starchless Chlamydomonas reinhardtii. Eukaryot Cell 8: 1856–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warakanont J, Tsai CH, Michel EJS, Murphy GR III, Hsueh PY, Roston RL, Sears BB, Benning C (2015) Chloroplast lipid transfer processes in Chlamydomonas reinhardtii involving a TRIGALACTOSYLDIACYLGLYCEROL 2 (TGD2) orthologue. Plant J 84: 1005–1020 [DOI] [PubMed] [Google Scholar]
- Work VH, Radakovits R, Jinkerson RE, Meuser JE, Elliott LG, Vinyard DJ, Laurens LML, Dismukes GC, Posewitz MC (2010) Increased lipid accumulation in the Chlamydomonas reinhardtii sta7-10 starchless isoamylase mutant and increased carbohydrate synthesis in complemented strains. Eukaryot Cell 9: 1251–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Shen H, Wang N, Tatlay J, Li L, Tan TW, Lee YK (2017) Elevated acetyl-CoA by amino acid recycling fuels microalgal neutral lipid accumulation in exponential growth phase for biofuel production. Plant Biotechnol J 15: 497–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zones JM, Blaby IK, Merchant SS, Umen JG (2015) High-resolution profiling of a synchronized diurnal transcriptome from Chlamydomonas reinhardtii reveals continuous cell and metabolic differentiation. Plant Cell 27: 2743–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]