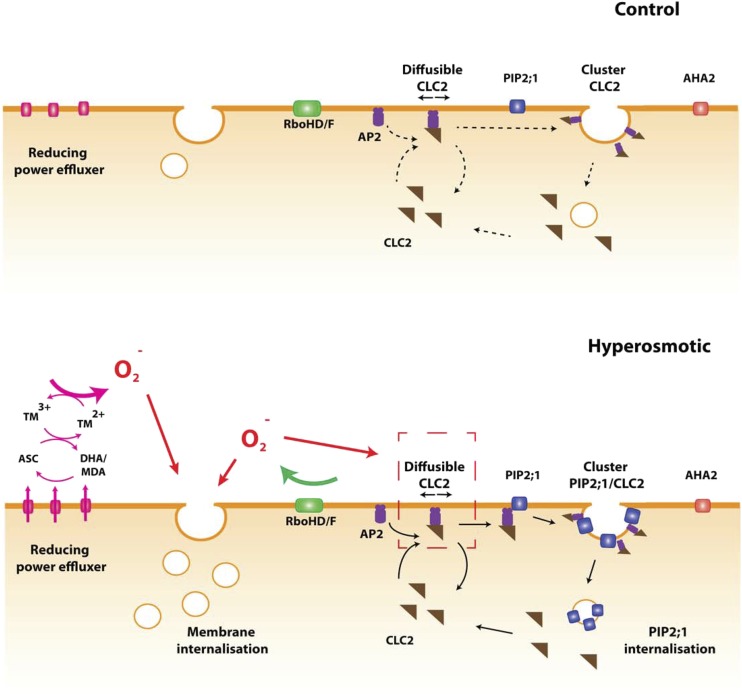
Figure 6.
Diagram of ROS signaling and its impact on protein dynamics after a hyperosmotic treatment. In control conditions, RBOHD/F (green) and putative efflux machinery (pink) for reducing power are inactive. A basal level of membrane internalization exists. CLC2 (brown) is either associated with AP2 (purple) in its diffusible form or is associated with CCVs, yielding nondiffusible forms. PIP2;1 (blue) and AHA2 (red) are organized in clusters and are mostly immobile. After a hyperosmotic treatment, activation of RBOHD/F and the efflux machinery (pink) for reducing power leads to enhanced production of superoxide. In the case of the reducing power efflux, this is achieved either by a direct efflux of cytoplasmic Asc or an efflux or regeneration of DHA. The resulting reducing power reduces apoplastic transition metals, which in turn react with oxygen to generate ROS. The accumulation of ROS enhances lipid membrane internalization by an unknown mechanism. In parallel, ROS produced by RBOH facilitate PM association of CLC2/AP2 complexes by promoting interactions of AP2 with lipid at the membrane. An excess of these complexes can bind to PIP2;1, thereby facilitating its incorporation in CCVs. As a consequence, PIP2;1 clustering and endocytosis are enhanced. The rate of CLC2 dissociation from the CCV is intrinsically enhanced by the hyperosmotic stress, and this effect is compensated for by RBOH-dependent ROS and the above-mentioned effects on CL2/AP2 complex formation.