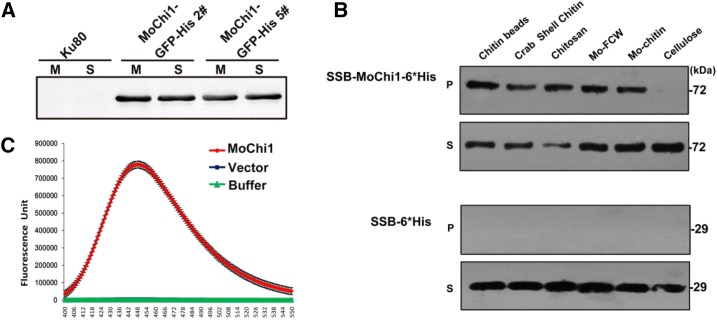
Figure 2.
Chitinolytic features of MoChi1. A, Secretion assay of MoChi1 fusion proteins from M. oryzae. M, Mycelia; S, supernatants. B, Polysaccharide affinity precipitation assay for recombinant SSB-MoChi1-6*His protein. At top, 10−3 μmol of MoChi1 coprecipitated with insoluble chitin magnetic beads, crab shell chitin, chitosan, fungal cell wall from M. oryzae (Mo-FCW), and colloidal chitin from M. oryzae (Mo-chitin) in insoluble pellet (P) and supernatant (S) fractions. At bottom, mock vector pSSBE1 coding SSB-6*His was used as the control. C, MoChi1 could degrade chitin in vitro. Short-chain fluorescent substrate MUC3 was used for chitiolytic activity. The 4-muthylumbelliferone released by MoChi1 was then detected with excitation wavelength of 360 nm and emission wavelength of 448 nm. Mock SSB-6*His protein and incubation buffer were used as controls. The enzymatic activity is presented as relative fluorescence units.