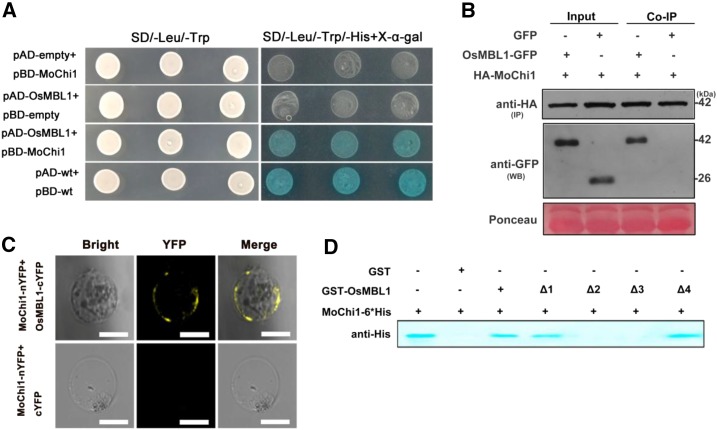
Figure 5.
OsMBL1 interacts with Mochi1 in vitro and in vivo. A, Y2H assay between pBD-MoChi1 and pAD-OsMBL1. Yeast cells were plated on SD/-Leu/-Trp and SD/-Leu/-Trp/-His + 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside acid (X-α-gal). pBD-wt and pAD-wt were used as positive controls by expressing wild-type fragment C of lambda cI repressor (aa 132-236). B, HA agarose bases Co-IP assay indicating interaction between HA-MoChi1 and OsMBL1-GFP in N. benthamiana leaves. Mock GFP was used as a negative control. HA-MoChi1 proteins were immunoprecipitated (IP) with anti-HA agarose beads. The GFP tagged OsMBL1 (OsMBL1-GFP) was detected by antibody anti GFP in western blotting (WB). Top and middle images represent immunoblot detection with anti-HA and anti-GFP antibodies, respectively; bottom image shows Ponceau S staining for equal loading. C, BiFC assay in rice protoplast cells. Top, Coexpression of MoChi1-nYFP with OsMBL1-cYFP; bottom, coexpression of MoChi1-nYFP with mock c-YFP. Bars = 50 μm. D, GST pull-down assay for the interaction of OsMBL1 and MoChi1. Full-length OsMBL1 (amino acids 1–145) and four truncated versions of OsMBL1 (Δ1, amino acids 20-145; Δ2, amino acids 1-80; Δ3, amino acids 20-80; and Δ4, amino acids 80-145) fusion proteins were precipitated with Glutathione-Sepharose beads and assayed for detecting the interaction with SSB-MoChi1-6*His proteins. GST was used as a negative control. An antibody against His was used to detect SSB-MoChi1-6*His fusion proteins.