Abstract
Park and Woo outline the cellular events leading to failure of the insulin-producing β cells in type 2 diabetes.
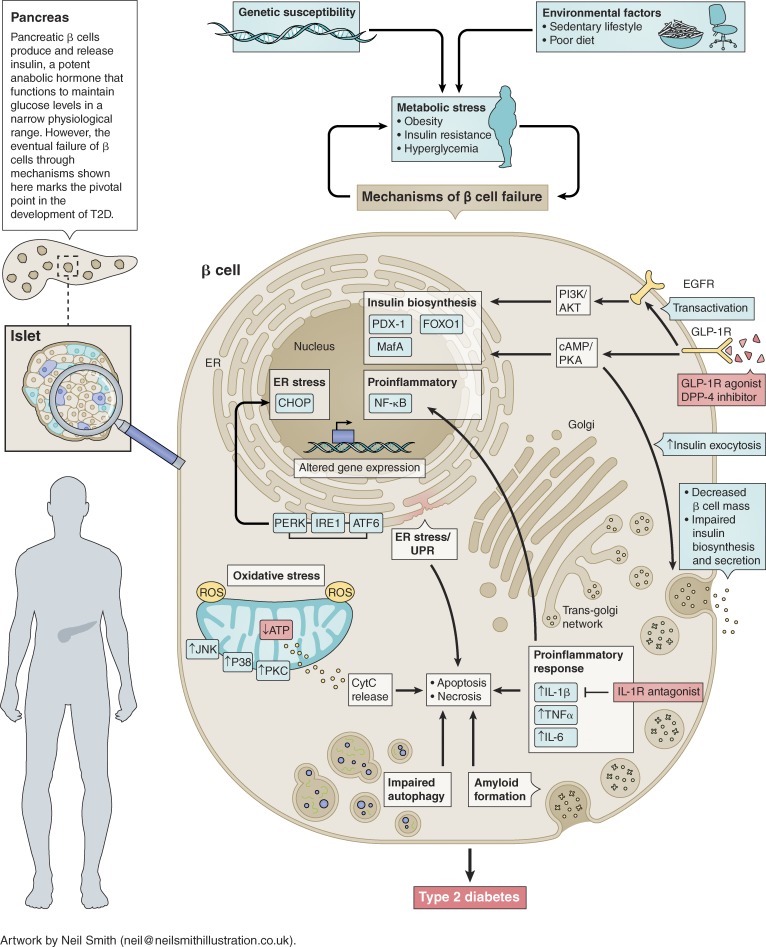
Insulin is a potent anabolic hormone that mediates glucose uptake in insulin-responsive tissues such as muscle, fat, and liver while inhibiting hepatic glucose production to maintain euglycemia. Type 2 diabetes (T2D) occurs when insulin-producing β cells of pancreatic islets are unable to produce and/or release sufficient insulin to overcome peripheral insulin resistance, resulting in hyperglycemia. Multiple metabolic and inflammatory cellular stresses ultimately lead to the impaired insulin biosynthesis and secretion from β cells in response to glucose, culminating in β cell failure. These include oxidative and ER stress, proinflammatory responses, and islet amyloid deposition, which can lead to β cell apoptosis or necrosis. Deteriorating β cell function also leads to impaired insulin gene expression by down-regulating insulin gene promoter activity, resulting in the eventual net decrease in insulin secretion in response to glucose stimulation.
In T2D, increased metabolic stress due to hyperglycemia and peripheral insulin resistance can induce mitochondrial dysfunction, leading to production of reactive oxygen species (ROS). Elevated levels of ROS can cause oxidative stress and activation of mitochondrial cytochrome C (CytC)–mediated apoptotic pathway in β cells. β cells inherently express low levels of antioxidant enzymes, rendering them prone to ROS-induced oxidative stress. Increased ROS activate multiple transcriptional pathways including c-Jun N-terminal kinase (JNK), p38, and PKC that can reduce insulin gene expression through suppression of pancreatic and duodenal homeobox 1 (PDX-1) and MafA binding to the insulin gene promoter (1). Moreover, transient oxidative stress down-regulates respiratory chain proteins and reduces mitochondrial ATP production, which can impair insulin secretion (2).
During development of T2D, peripheral insulin resistance can induce hyperinsulinemia through up-regulation of insulin transcription and translation, which can lead to the accumulation of unfolded or misfolded proteins in the β cell ER lumen. The overload of defective proteins can trigger the unfolded protein response (UPR) through activation of PKR–like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6) pathways, resulting in attenuation of global protein synthesis to promote proper protein folding and degradation of misfolded proteins. However, when UPR can no longer attenuate the increasing protein load on the ER, ER stress–mediated β cell dysfunction and death can occur (3).
Autophagy is the basic catabolic mechanism to degrade dysfunctional proteins as well as defective cellular components. During development of T2D, autophagy is enhanced to adapt to the dynamic changes occurring in β cells, in part providing protection against accumulating oxidative and ER stress (4). However, persistent metabolic stress on β cells under hyperglycemic conditions leads to dysregulation of autophagy, which can ultimately aggravate β cell function or result in β cell demise.
Islet inflammation, characterized by immune cell infiltration by macrophages, can lead to local release of cytokines and chemokines. This, in turn, can activate nuclear factor κ–light-chain-enhancer of activated B cells (NF-κB)–mediated transcriptional up-regulation of proinflammatory genes, contributing to β cell dysfunction and death (5). Increased islet inflammation and islet-associated macrophages have been detected in pancreatic sections from humans with T2D and in animal models of T2D. Moreover, islet β cells themselves can also produce cytokines and chemokines such as IL-1β, IFNγ, TNFα, and IL-6 (6). These proinflammatory signals can in turn activate apoptotic mechanisms including ER stress and UPR in β cells.
Islet amyloid is a pathological lesion found in the pancreas of more than 90% of individuals with T2D (7). Islet amyloid is formed mainly by abnormal aggregation of the β cell hormone islet amyloid polypeptide, which is coproduced and cosecreted with insulin. Increased islet amyloid deposition correlates with decreased β cell mass and insulin production via multiple mechanisms including promotion of islet inflammation and activation of apoptotic pathways (8, 9).
Taken together, deteriorating pancreatic β cell function and mass through multiple mechanisms (see illustration) promote hyperglycemia due to inadequate levels of circulating insulin, ultimately resulting in T2D. Preservation of β cell mass and function is therefore critical to maintain glucose homeostasis and prevent diabetes. Pharmacological agents currently used for treatment of T2D include incretin-based therapies such as GLP-1 receptor (GLP-1R) agonists or dipeptidyl peptidase-4 (DPP-4; enzyme responsible for GLP-1 inactivation) inhibitors, which can enhance β cell proliferation and function through activation of multiple signaling pathways including the epidermal growth factor receptor (EGFR)–mediated PI3K/Akt signaling and the cAMP/PKA–dependent signaling pathways (10). Better understanding of the underlying mechanisms contributing to β cell loss and dysfunction will facilitate the identification of novel therapeutic targets to effectively restore and maintain β cell physiology for improved glycemic control.
Acknowledgments
This work was supported by grants to M. Woo from the Canadian Institutes of Health Research (CIHR) MOP 142193. M. Woo holds a Canada Research Chair in Signal Transduction in Diabetes Pathogenesis. Y.J. Park is the recipient of postdoctoral fellowships from CIHR, Diabetes Canada, and Banting and Best Diabetes Centre.
The authors declare no competing financial interests.
References
- 1.Juliana C.A., et al. . 2017. Proc. Natl. Acad. Sci. USA. 114:1341–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim-Muller J.Y., et al. . 2016. Nat. Commun. 7:12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song B., et al. . 2008. J. Clin. Invest. 118:3378–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee M.S. 2014. Trends Endocrinol. Metab. 25:620–627. [DOI] [PubMed] [Google Scholar]
- 5.Nordmann T.M., et al. . 2017. Sci. Rep. 7:6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boni-Schnetzler M., et al. . 2008. J. Clin. Endocrinol. Metab. 93:4065–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jurgens C.A., et al. . 2011. Am. J. Pathol. 178:2632–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park Y.J., et al. . 2014. Diabetologia. 57:765–775. [DOI] [PubMed] [Google Scholar]
- 9.Masters S.L., et al. . 2010. Nat. Immunol. 11:897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drucker D.J., et al. . 2018. Cell Metab. 27:740–756. [DOI] [PubMed] [Google Scholar]