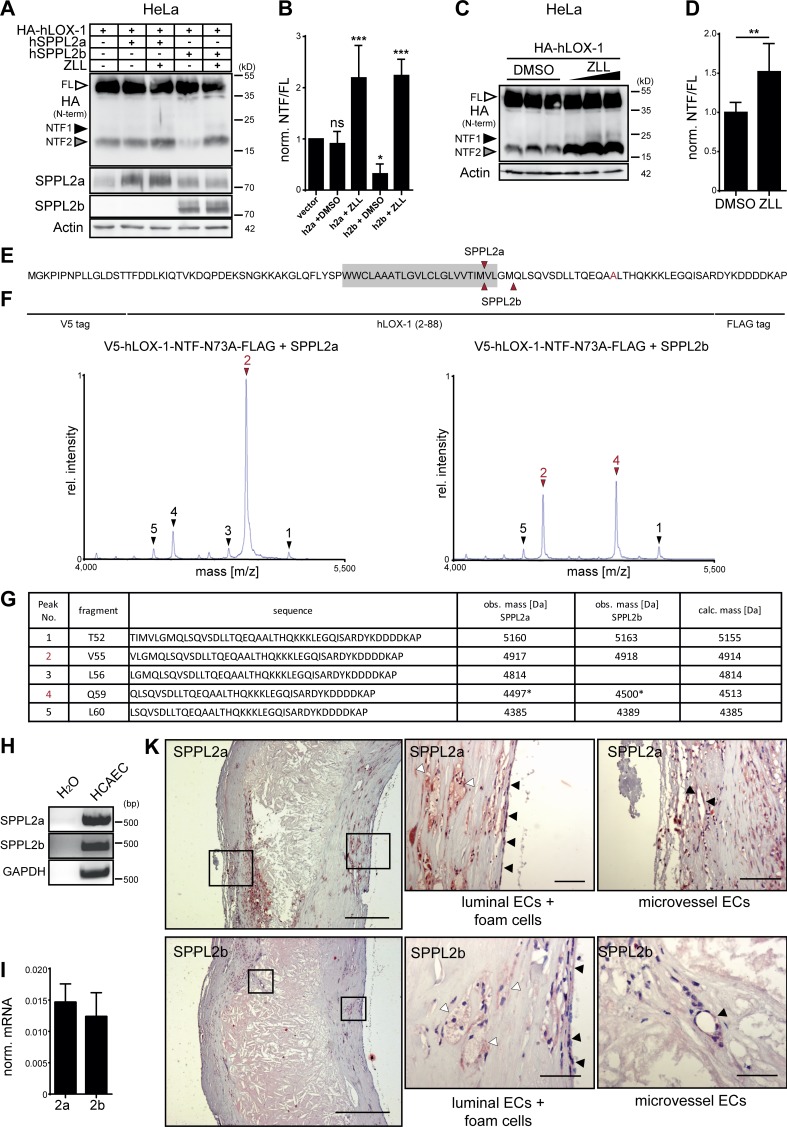
Figure 9.
The role of SPPL2a/b for processing of LOX-1 NTFs is conserved in humans. (A) HeLa cells were transfected with HA-tagged hLOX-1 alone or in combination with human SPPL2a (hSPPL2a) or SPPL2b (hSPPL2b-myc). Where indicated, cells were treated with 20 µM ZLL for 6 h before lysis and Western blot analysis. (B) Quantification of A. N = 3–4, n = 3–4. One-way ANOVA with Dunnett’s post hoc test. (C) Accumulation of hLOX-1 NTFs in HeLa cells upon inhibition of endogenous SPPL2 proteases with 10, 20, and 40 µM ZLL. (D) Quantification of C for 40 µM ZLL treatment. N = 4, n = 8. Student’s t test. (E–G) The cleavage sites of human SPPL2a and SPPL2b in the TMD of hLOX-1 were analyzed by MS using V5-hLOX-1 NTF N73A-FLAG as model substrate. Arrows indicate peptides up-regulated by protease overexpression (red, dominant peaks). Peak 4 corresponds to a peptide starting at Q59 with a potential glutamate to pyroglutamate conversion. Rel., relative. (H and I) SPPL2a and SPPL2b are expressed in human coronary artery endothelial cells (HCAECs) as revealed by RT-PCR (H) and qRT-PCR (I). (K) Presence of SPPL2a and SPPL2b in human atherosclerotic plaques was investigated by immunohistochemistry. Bars, 400 µm; close-ups, 50 µm. ***, P ≤ 0.001; **, P ≤ 0.01; *, P ≤ 0.05; ns, not significant. N, the number of independent experiments; n, the number of individual samples for quantification. All data are shown as mean ± SD.