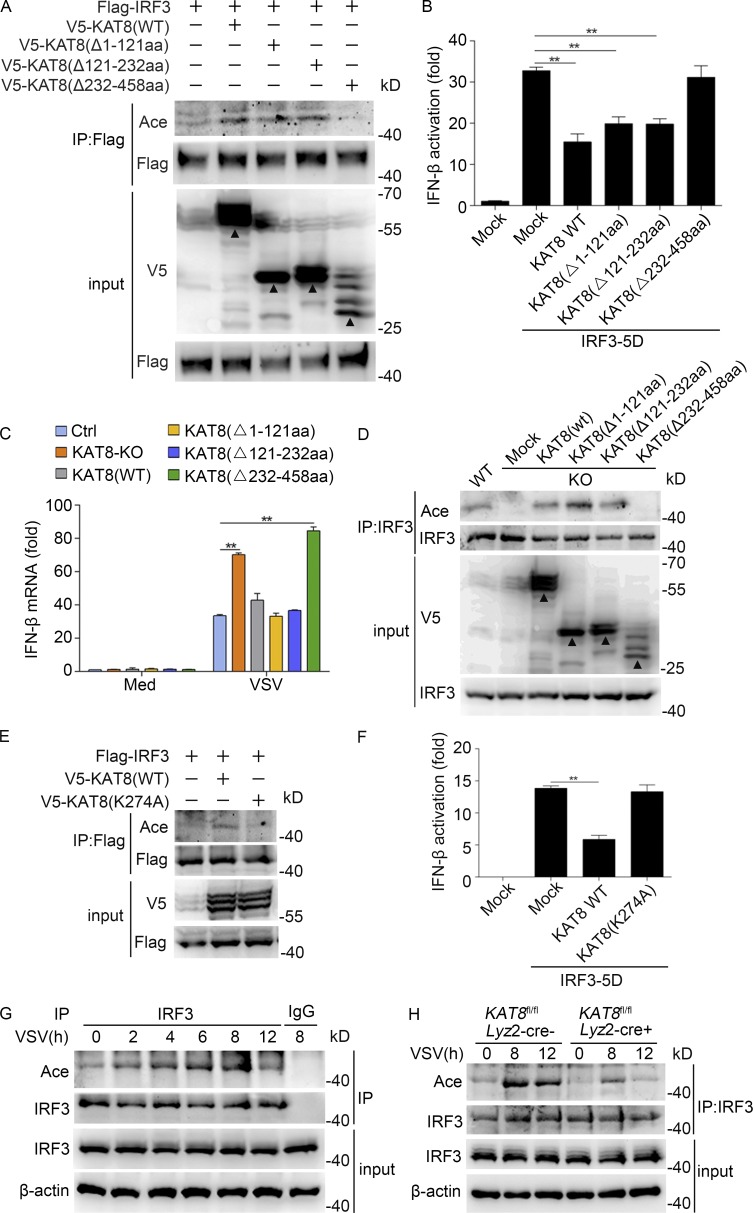
Figure 5.
KAT8 promotes IRF3 acetylation via its MYST domain. (A) Immunoblot analysis of acetylation in HEK293T cells transiently transfected with V5-tagged WT or mutant KAT8 plus Flag-tagged IRF3 and assessed 24 h later before (input) or after IP with antibody to Flag. (B) Luciferase activity of an IFN-β reporter in HEK293T cells transfected with WT KAT8 or KAT8 truncations together with IRF3-5D. (C) KAT8-KO RAW264.7 cells were transfected with WT KAT8 or KAT8 truncations and then infected 24 h later with VSV (1 MOI) for 8 h. The expression of IFN-β at the mRNA level was measured by Q-PCR. (D) Immunoblot analysis of IRF3 acetylation and total IRF3 in KAT8-KO RAW264.7 cells transiently transfected with V5-tagged WT or mutant KAT8 and then infected 24 h later with VSV (1 MOI) for 8 h, assessed before (input) or after IP with antibody to IRF3. (E) Immunoblot analysis of acetylation in HEK293T cells transiently transfected with V5-tagged WT or mutant KAT8 plus Flag-tagged IRF3 and assessed 24 h later before (input) or after IP with antibody to Flag. (F) Luciferase activity of an IFN-β reporter in HEK293T cells transfected with WT KAT8 or KAT8 mutant (K274A) together with IRF3-5D. (G) Immunoblot analysis of endogenous acetylation of IRF3 in peritoneal macrophages infected for the indicated times with VSV (1 MOI), assessed before (input) or after IP with IgG or antibody to IRF3. (H) Immunoblot analysis of IRF3 acetylation in KAT8fl/flLyz2-Cre− or KAT8fl/flLyz2-Cre+ peritoneal macrophages infected for the indicated times with VSV (1 MOI), assessed before (input) or after IP with antibody to IRF3. **, P < 0.01 (one-way ANOVA; B and F); **, P < 0.01 (two-tailed Student’s t test; C). Data are representative of three independent experiments with similar results (A, D, E, G, and H) or are from three independent experiments (B, C, and F; mean ± SEM). Arrowheads, specific bands.