Table 1.
Selective Boc-protection of 4.a
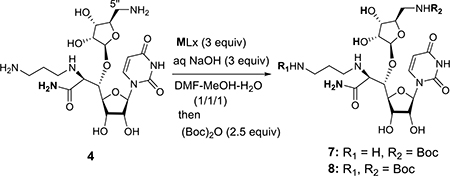 | ||||
|---|---|---|---|---|
| Entry | MLx | Time (h) | Selectivity (7 : 8)c | Yield for 7 (%)d |
| 1 | CuSO4 | 12 | 1 : 0 | 20 |
| 2 | Cu(OAc)2 | 12(1)b | 1 : 0 | 95 |
| 3 | CuBr2 | 12 | 1 : 0 | 10 |
| 4 | CuCl2 | 12(5)b,e | 1 : 0 | 90 |
| 5 | NiCl2•6H2O | 12(5)b | >19 : 1 | 85 |
| 6 | Ni(OAc)2•nH2O | 12(5)b | >19 : 1 | 85 |
| 7 | Ni(NO3)2•6H2O | 12(5)b | >19 : 1 | 85 |
| 8 | VCl3 | 12 | 4 : 1 | 40 |
| 9 | Zn(NO3)2•6H2O | 12(3)b | 1 : 1 | 45 |
| 10 | ZnCl2 | 12(3)b | 1 : 1 | 40 |
| 11 | CoCl2 | 12(3)b | 1 : 1 | 45 |
| 12 | FeSO4•H2O | 12(3)b | 1 : 1 | 40 |
| 13 | FeCl3•6H2O | 12(3)b | 1 : 1 | 40 |
| 14 | - | 12(1)b | 1 : 1 | 35 |
Reaction condition: 3 (1.0 equiv), MLx (3.0 equiv), and NaOH (1N, 4.0 equiv) in DMF-MeOH-H2O (1/1/1, 0.05M), after 30 min. (Boc)2O (2.5 equiv)
time in the parenthesis: time required for the reaction to be completed
Selectivity was determined via HPLC analysis for the reaction mixtures after 12h
Isolated yield
the same reaction with CuCl2 (1.0 equiv) provided 6 in >90 yield in 1 h.
