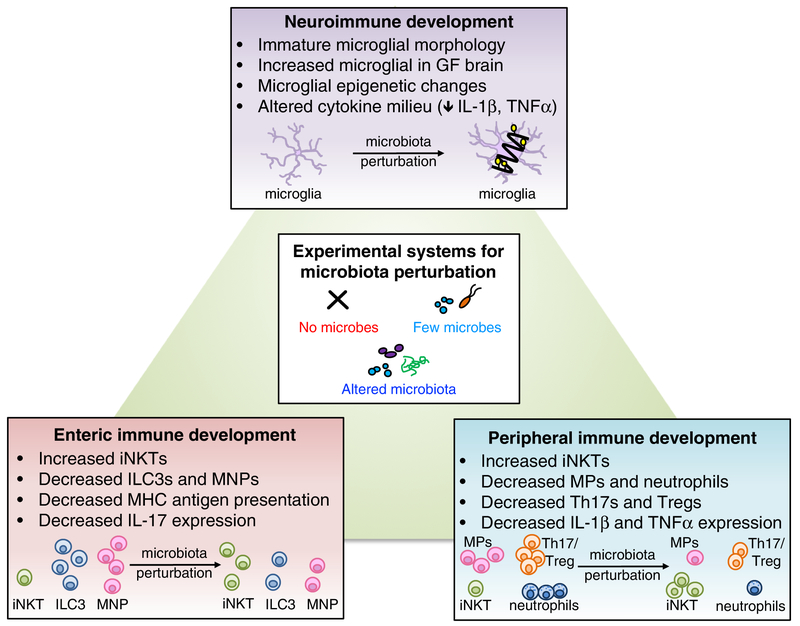
Figure 1. Roles for the microbiome in neuro-, peripheral and enteric immune development.
Microbiota perturbations, including elimination, reduction, or alteration of endogenous microbes, lead to altered immune development in multiple tissue sites. In the brain, absence of the microbiome alters morphological and transcriptional features of brain-resident microglia. Additionally, the cytokine milieu of the brain is altered in the absence of microbes. In the periphery, particular bacteria such as Lactobacillus modulate memory T cells, and microbial metabolites such as the short-chain fatty acid, butryate, regulate Treg cell populations. Depletion of the microbiome results in peripheral immune dysfunction, reducing macrophages (MP) and neutrophils and increasing iNKT cells. Altered diversity of the microbiota also impacts immune development. When microbes are substantially reduced, both Th17 and Treg cell numbers decrease. Conversely, alteration of microbiota composition, as with increased prevalence of Bacteroides fragilis, increases Th17 and Treg cells. In the intestine, transient maternal colonization with non-replicating E. coli, or with altered Schaedler flora, increases ILC3s, while ablation of the microbiota reduces gut ILC3s, mononuclear phagocytes, Paneth cell activity, MHC antigen presentation, and mast cell gene expression.